According To The Ph Chart Which Is The Strongest Base
According To The Ph Chart Which Is The Strongest Base - For any aqueous solution at 25 ∘ c. Some of the common strong acids and bases are listed here (credit: The blood is slight basic in nature and the ph of blood is. According to the chart, which is the strongest acid, based on its ph level? Chemistry (openstax), cc by 4.0 ). Convert the hydrogen ion concentration to a ph. However, for a very dilute strong acid solution with concentration less than 1 × 10 − 7m, the ph is dominated by the. Download the acid & base chart. Web a strong base yields 100% (or very nearly so) of oh − and hb + when it reacts with water; In solutions of the same concentration, stronger acids ionize to a greater extent, and so yield higher concentrations of hydronium ions than do weaker acids. Weakest 3.2 * 10 9: We can rank the strengths of bases by their tendency to form hydroxide ions in aqueous solution. Web order the following from weakest base to strongest base: For every factor of 10. Web the most common strong acids and bases are listed in table 1. A ph close to zero indicates a (n) _______ solution, and a ph near 14 indicates a (n) _______ solution. Figure 14.6 some of the common strong acids and bases are listed here. However, for a very dilute strong acid solution with concentration less than 1 × 10 − 7m, the ph is dominated by the. Ph + poh =. Buffers work by reacting with a base or acid to control the ph of a solution. Web for a strong acid, \ce[h +] = \ce[a −] = concentration of acid if the concentration is much higher than 1 × 10 − 7m. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Web. Some of the common strong acids and bases are listed here (credit: Naoh is a strong base whose ph is 14. A buffer solution contains a weak acid and its conjugate base or a weak base and its conjugate acid. List of common polyatomic ions. Web the ph scale runs from 0 to 14, and is an indication of how. Topics covered in other articles. Buffers work by reacting with a base or acid to control the ph of a solution. According to the ph chart, which is the strongest base? Web the most common strong acids and bases are listed in table 1. Which has the highest concentration of h+ ions: Ph + poh = 14. Download the acid & base chart. Web familiar solutions can have a wide range of ph levels. Soluble ionic hydroxides such as naoh are considered strong bases because they dissociate completely when dissolved in water. [ oh −] [ oh −] = 10 − poh. The relative strengths of acids may be determined by measuring their equilibrium constants in aqueous solutions. Web in aqueous solutions, \(h_3o^+\) is the strongest acid and \(oh^−\) is the strongest base that can exist in equilibrium with \(h_2o\). Naoh is a strong base whose ph is 14. Figure \(\pageindex{1}\) lists several strong bases. According to the ph chart, which is. According to the ph chart, which is the strongest base? A ph close to zero indicates a (n) _______ solution, and a ph near 14 indicates a (n) _______ solution. Lemon juice has approximately how many more times the h+ ions than tomato juice? Soluble ionic hydroxides such as naoh are considered strong bases because they dissociate completely when dissolved. The blood is slight basic in nature and the ph of blood is. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Topics covered in other articles. Download the acid & base chart. The relative strengths of acids may be determined by measuring their equilibrium constants in aqueous solutions. Web for a strong acid, \ce[h +] = \ce[a −] = concentration of acid if the concentration is much higher than 1 × 10 − 7m. Topics covered in other articles. For every factor of 10. Web familiar solutions can have a wide range of ph levels. Web in aqueous solutions, \(h_3o^+\) is the strongest acid and \(oh^−\) is the. Web a strong base yields 100% (or very nearly so) of oh − and hb + when it reacts with water; Web the most common strong acids and bases are listed in table 1. Web a weak acid gives small amounts of h3o+ and a −. In this tutorial, you will learn about the properties and ph of strong acids and bases, and how to calculate their ph, poh, pka, and pkb values. Web a strong base yields 100% (or very nearly so) of oh − and hb + when it reacts with water; Figure \(\pageindex{1}\) lists several strong bases. Use k w to work out the hydrogen ion concentration. Web for a strong acid, \ce[h +] = \ce[a −] = concentration of acid if the concentration is much higher than 1 × 10 − 7m. Figure 14.6 some of the common strong acids and bases are listed here. The relative strengths of acids may be determined by measuring their equilibrium constants in aqueous solutions. Acid base strength of acid: In solutions of the same concentration, stronger acids ionize to a greater extent, and so yield higher concentrations of hydronium ions than do weaker acids. Web 34 rows table of acid and base strength; Web the most common strong acids and bases are listed in figure 14.6. Ph + poh = 14. Soluble ionic hydroxides such as naoh are considered strong bases because they dissociate completely when dissolved in water.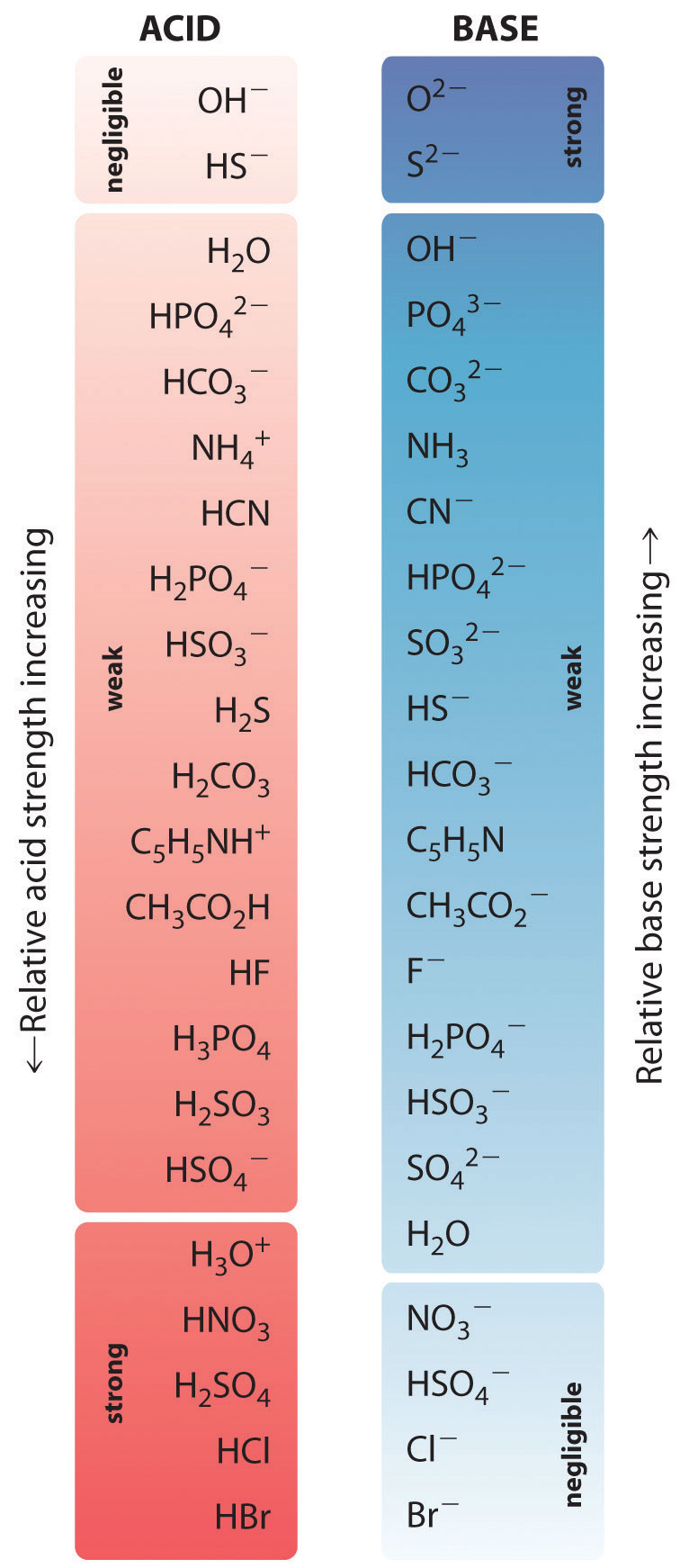
According To The Ph Chart Which Is The Strongest Base
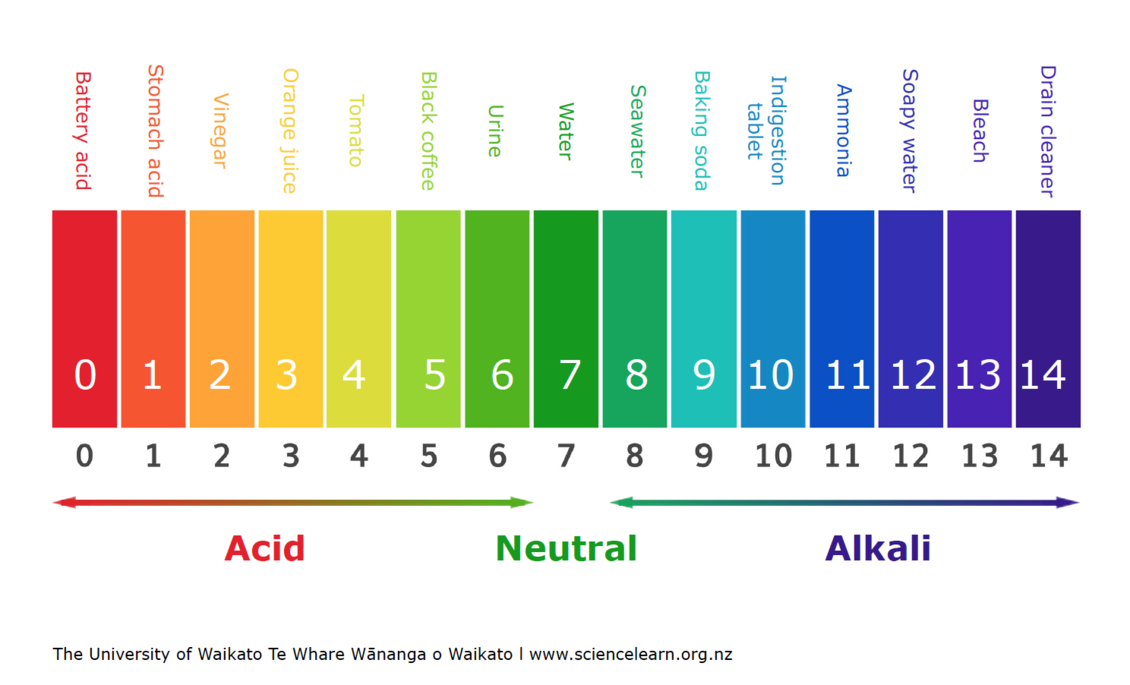
pH scale — Science Learning Hub

Strength of Acids and Bases (How to find it?) Chemistry Teachoo
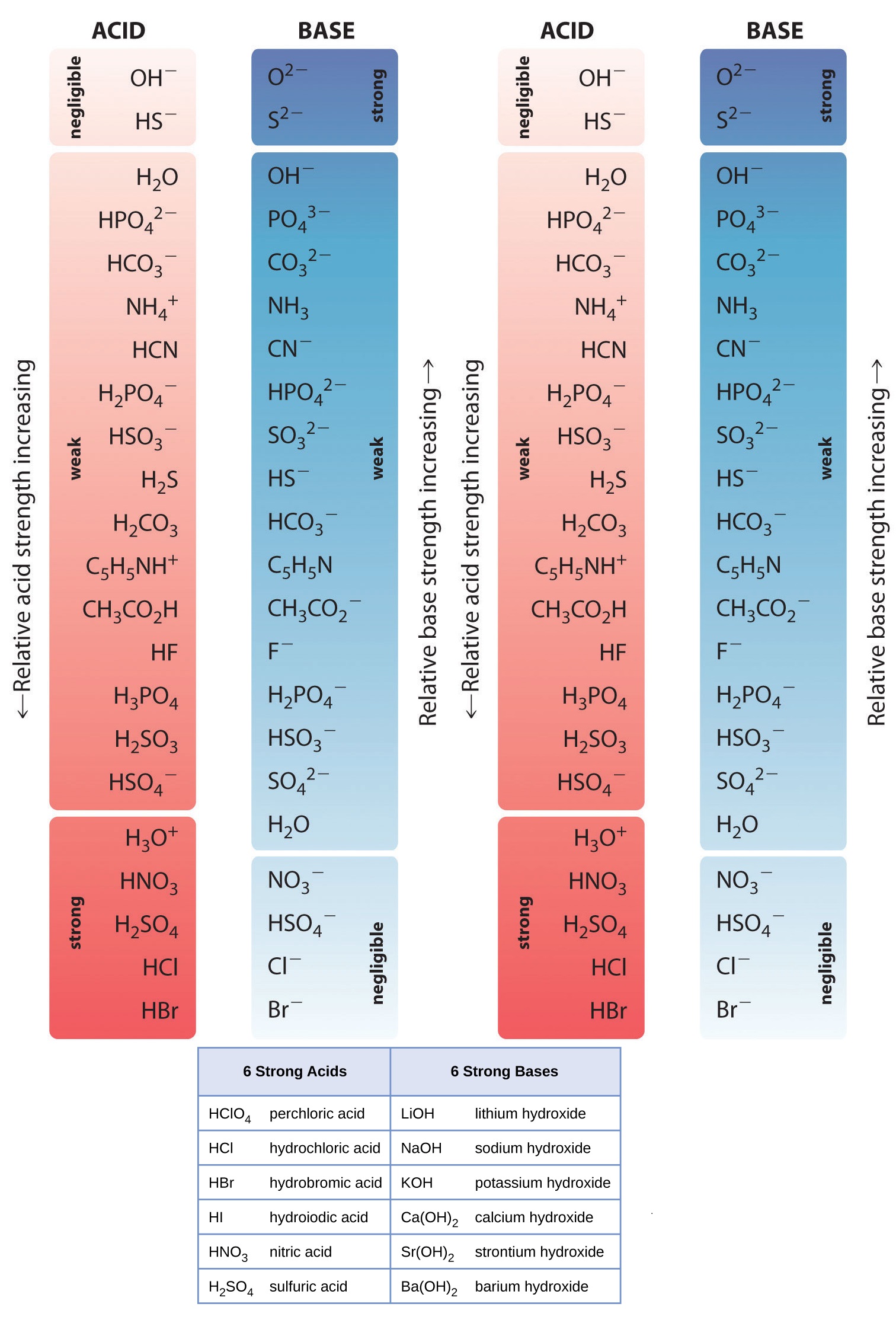
List of Strong Acids & Bases in Order StudyPK
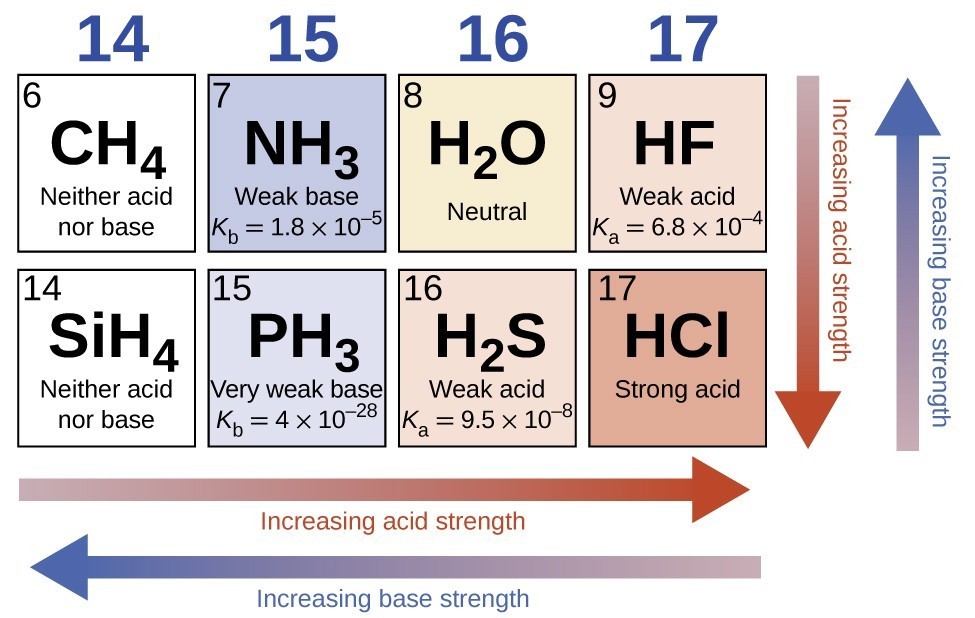
Relative Strengths of Acids and Bases Chemistry Atoms First

pH Of Acids And Bases Calculate pH Value Chemistry Byju's

Back to Basics Acids, Bases & the pH Scale Precision Laboratories
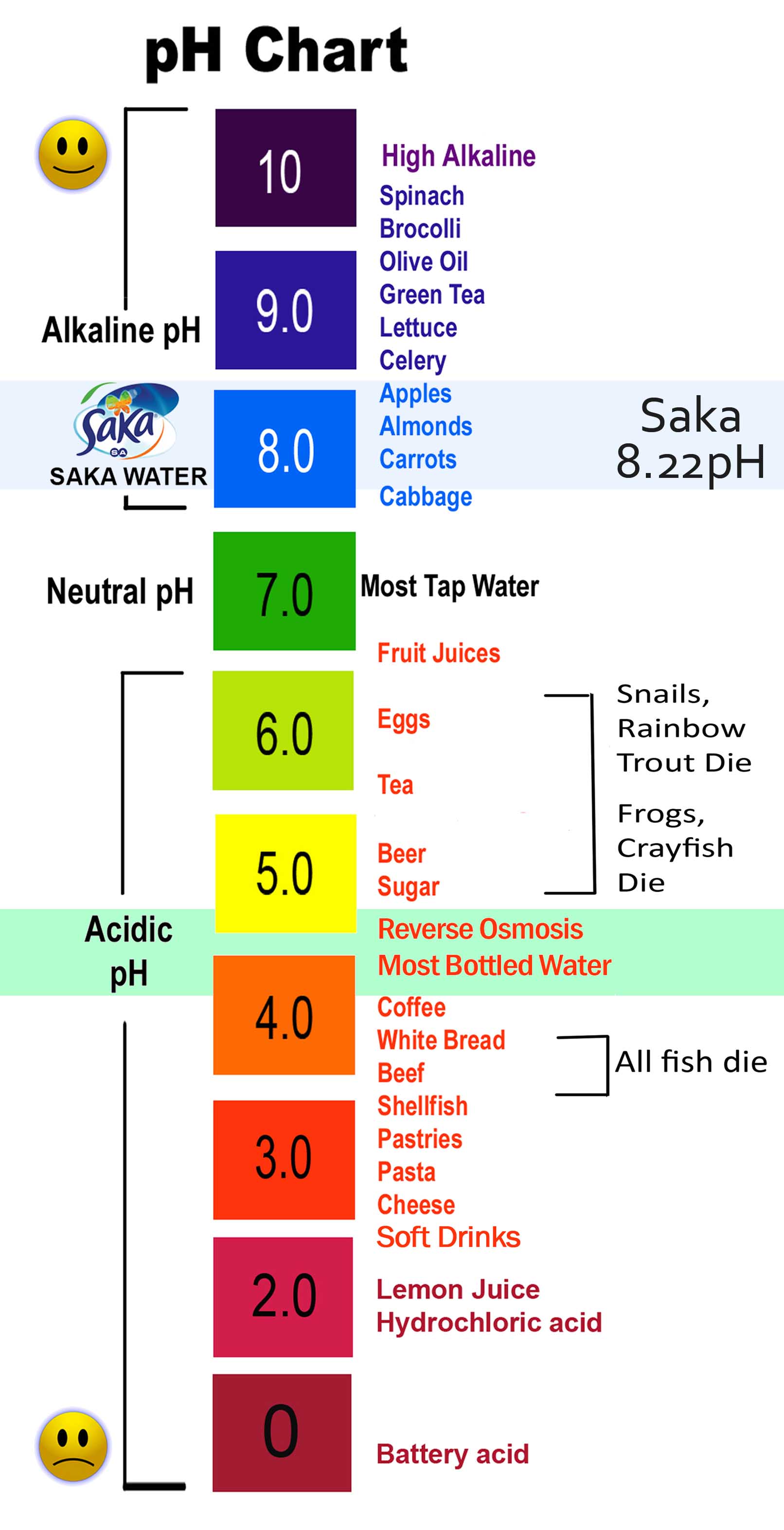
4a. Acids and Bases Antonia's chemistry blog

Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision
Acids and Bases CK12 Foundation
A Weak Base Yields A Small Proportion Of Hydroxide Ions.
Web The Ph Is A Measure Of The Concentration Of These.
Web In Aqueous Solutions, \(H_3O^+\) Is The Strongest Acid And \(Oh^−\) Is The Strongest Base That Can Exist In Equilibrium With \(H_2O\).
[ H +] [ H +] = 10 − Ph.
Related Post:
