Acid Strength Chart
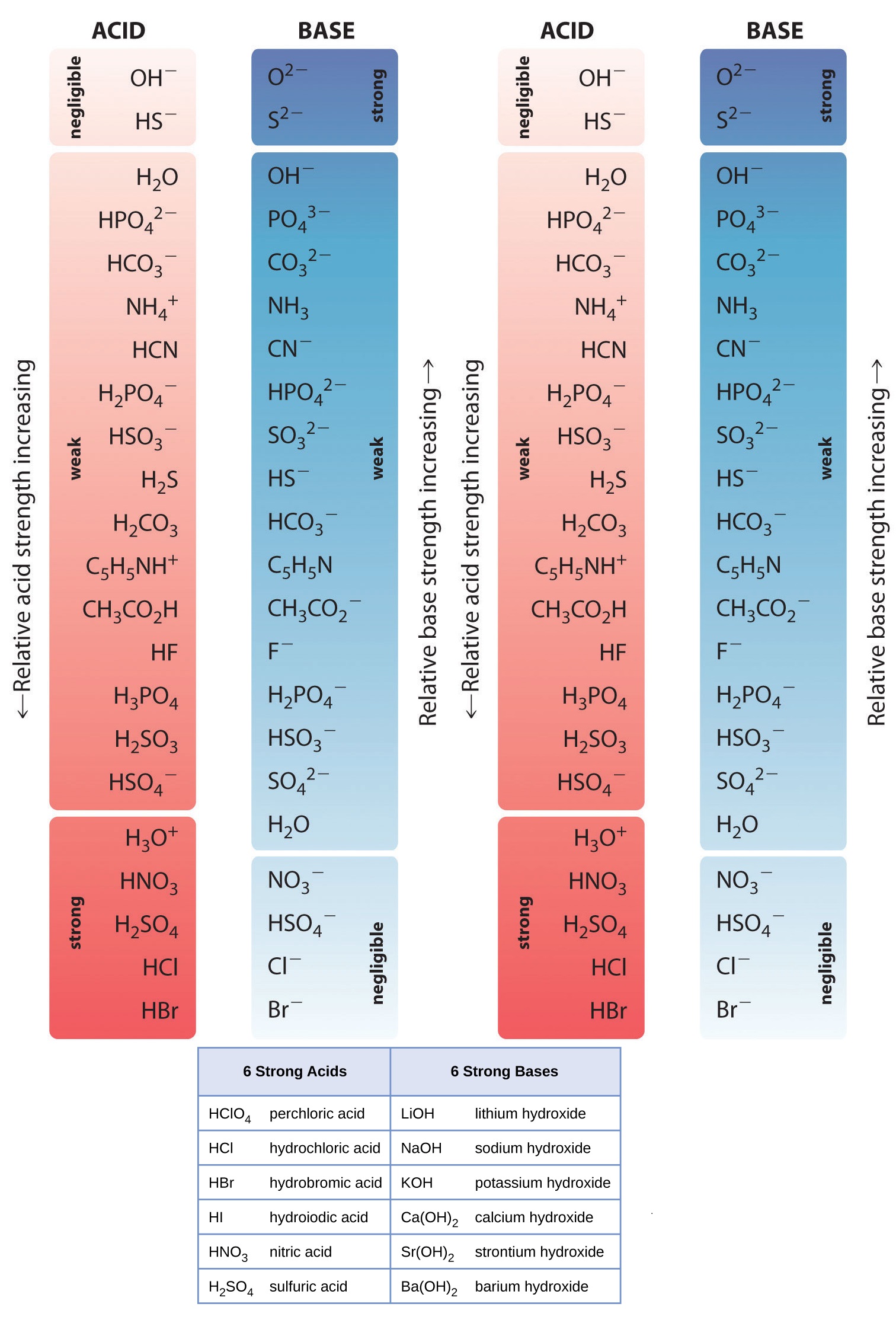
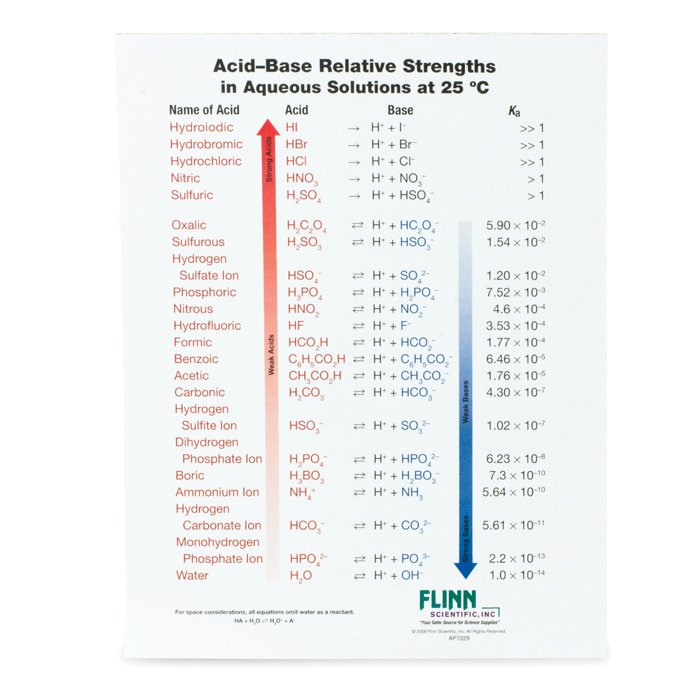
Acid Strength Chart - Web use this acids and bases chart to find the relative strength of the most common acids and bases. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. By andy brunning september 28, 2016. Web acids and bases behave differently in solution based on their strength. Look at where the negative charge ends up in each conjugate base. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. Web acid with values less than one are considered weak. In the ethyl anion, the negative charge is borne by carbon, while in the methylamine anion and methoxide anion the. The more stable (weaker) the conjugate base, the stronger the acid. Web figure 14.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. Web acids and bases behave differently in solution based on their strength. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. Web acid and base chart lists the. The first six acids in figure 14.3.3 are the most common strong acids. Examples of strong acids are hydrochloric acid. Acid strength increases down a group and increases from left to right across a period. Web a reactivity series, or acid strength chart, provides a visual representation of the relative strength of various acids. Web what’s the diference between acid. Web acids and bases behave differently in solution based on their strength. This information can be used to predict the outcome of reactions between acids and other substances, such as bases and metals. Examples of strong acids are hydrochloric acid. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. The dissociation of. Acid strength depends on a variety of chemical factors, including electronegativity, atomic radius, and resonance. The acid and base in a given row are conjugate to each other. Visit byju's to learn more about it. By andy brunning september 28, 2016. Web acid with values less than one are considered weak. By andy brunning september 28, 2016. Acid strength increases down a group and increases from left to right across a period. Keep reading to learn all about the chemistry behind acid strength. The more stable (weaker) the conjugate base, the stronger the acid. Acid or base strength is a measure of how readily the molecule ionizes in water. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. The first six acids in figure 14.3.3 are the most common strong acids. Visit byju's to learn more about it. In the ethyl anion, the negative charge is borne by carbon, while in the methylamine anion and methoxide anion the.. Topics covered in other articles. Acid or base strength is a measure of how readily the molecule ionizes in water. This graphic explains the basics. Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: Acid or base strength is a measure of how readily the molecule ionizes in water. The more stable (weaker) the conjugate base, the stronger the acid. While the weak acid can be estimated via the equilibrium constant ( keq ), it is constant for a dilute solution, and the. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Examples of strong acids are hydrochloric acid.. The first six acids in figure 14.3.3 are the most common strong acids. Web what’s the diference between acid strength and concentration? Web figure 14.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. While the weak acid can be estimated via the equilibrium constant. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). Acid and base ionization constants. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. In the ethyl anion, the negative charge is borne by carbon, while in the methylamine anion and methoxide. While the weak acid can be estimated via the equilibrium constant ( keq ), it is constant for a dilute solution, and the. Look at where the negative charge ends up in each conjugate base. Examples of strong acids are hydrochloric acid. They’re routinely described as strong or weak, concentrated or dilute. Web acid and base chart lists the strength of acids and bases (strongest to weakest) in order. This graphic explains the basics. Web use this acids and bases chart to find the relative strength of the most common acids and bases. In the ethyl anion, the negative charge is borne by carbon, while in the methylamine anion and methoxide anion the. Keep reading to learn all about the chemistry behind acid strength. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. Visit byju's to learn more about it. The acid and base in a given row are conjugate to each other. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). Simple to use laboratory reference chart for scientists, researchers and lab technicians. Acid strength depends on a variety of chemical factors, including electronegativity, atomic radius, and resonance. Table \(\pageindex{1}\) lists several strong acids.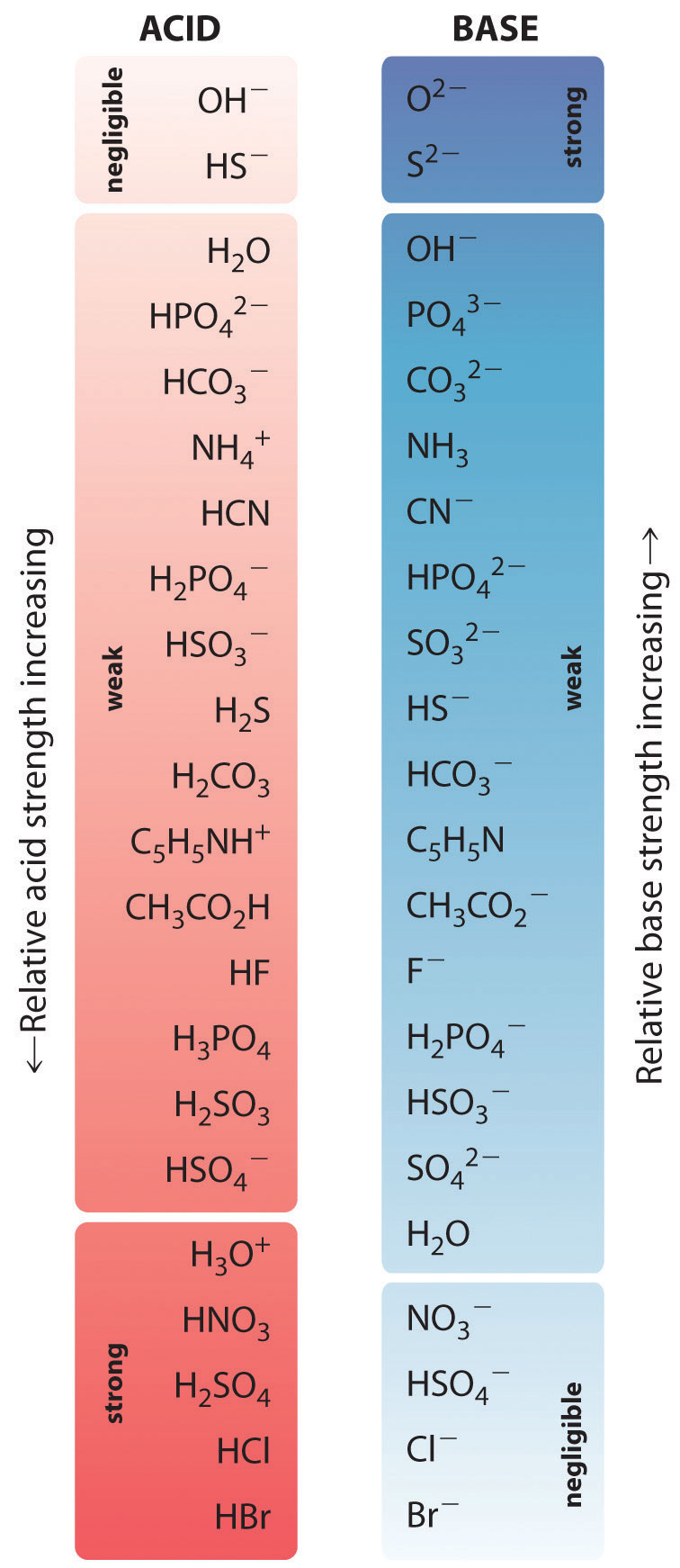
Acid Strengths Table
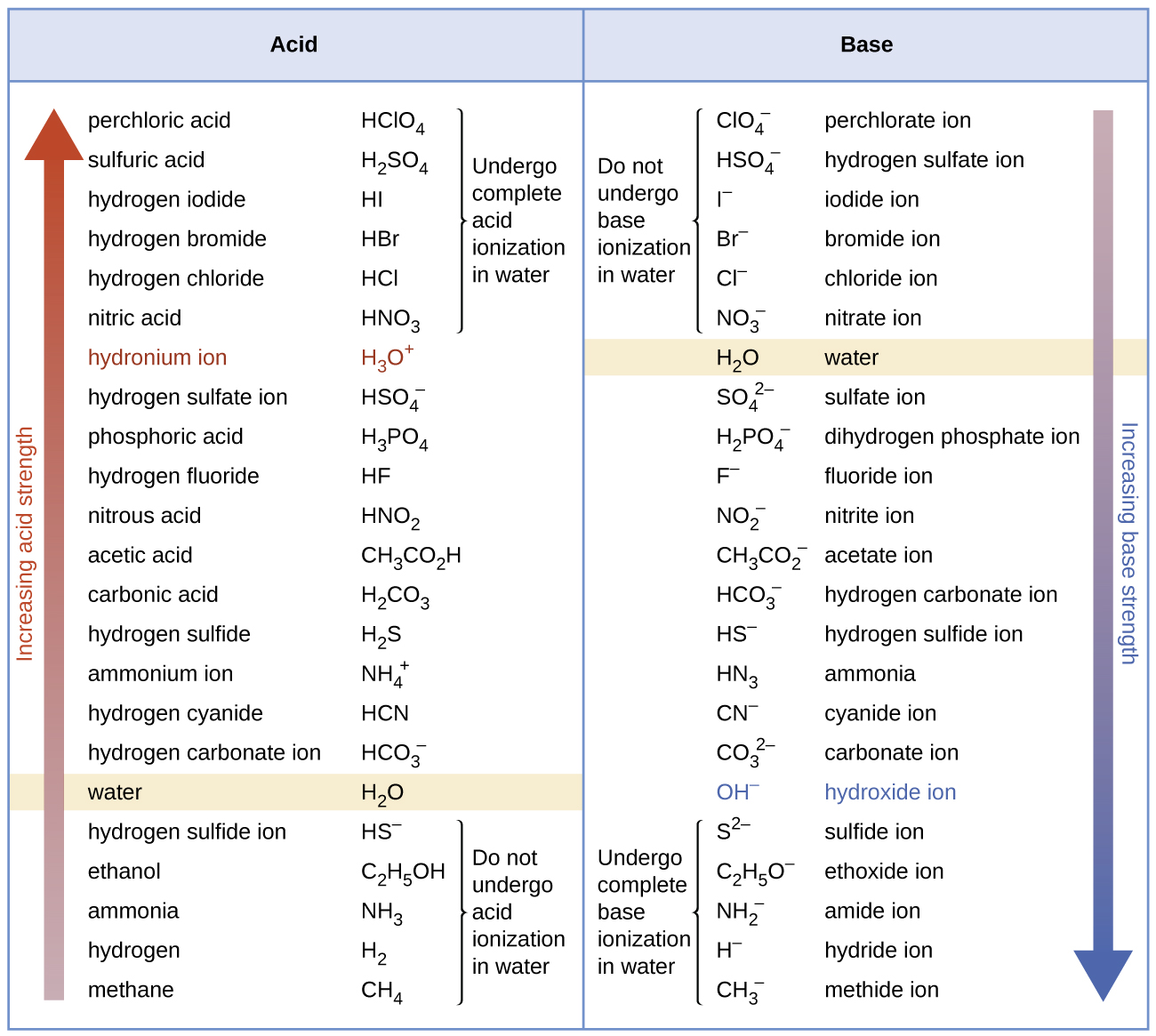
14.3 Relative Strengths of Acids and Bases Chemistry 112 Chapters 12
List of Strong Acids & Bases in Order StudyPK
AcidBase Strength Charts for Chemistry

Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision
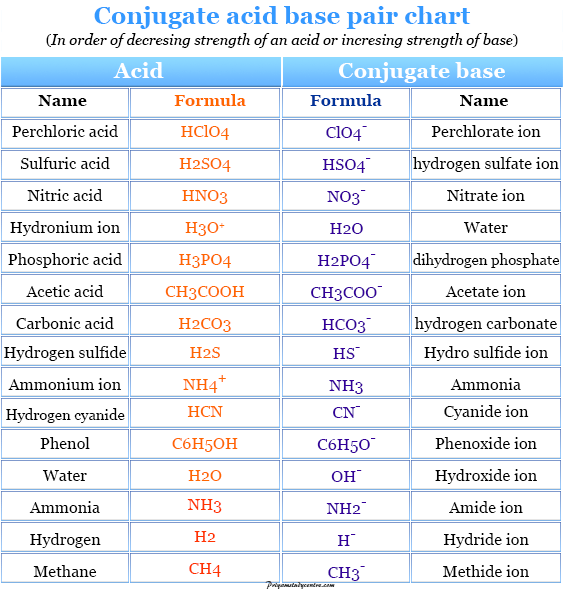
Conjugate Acid Base pair Definition, Concept, Examples, List
pKa Values and strengths of Acids and Bases
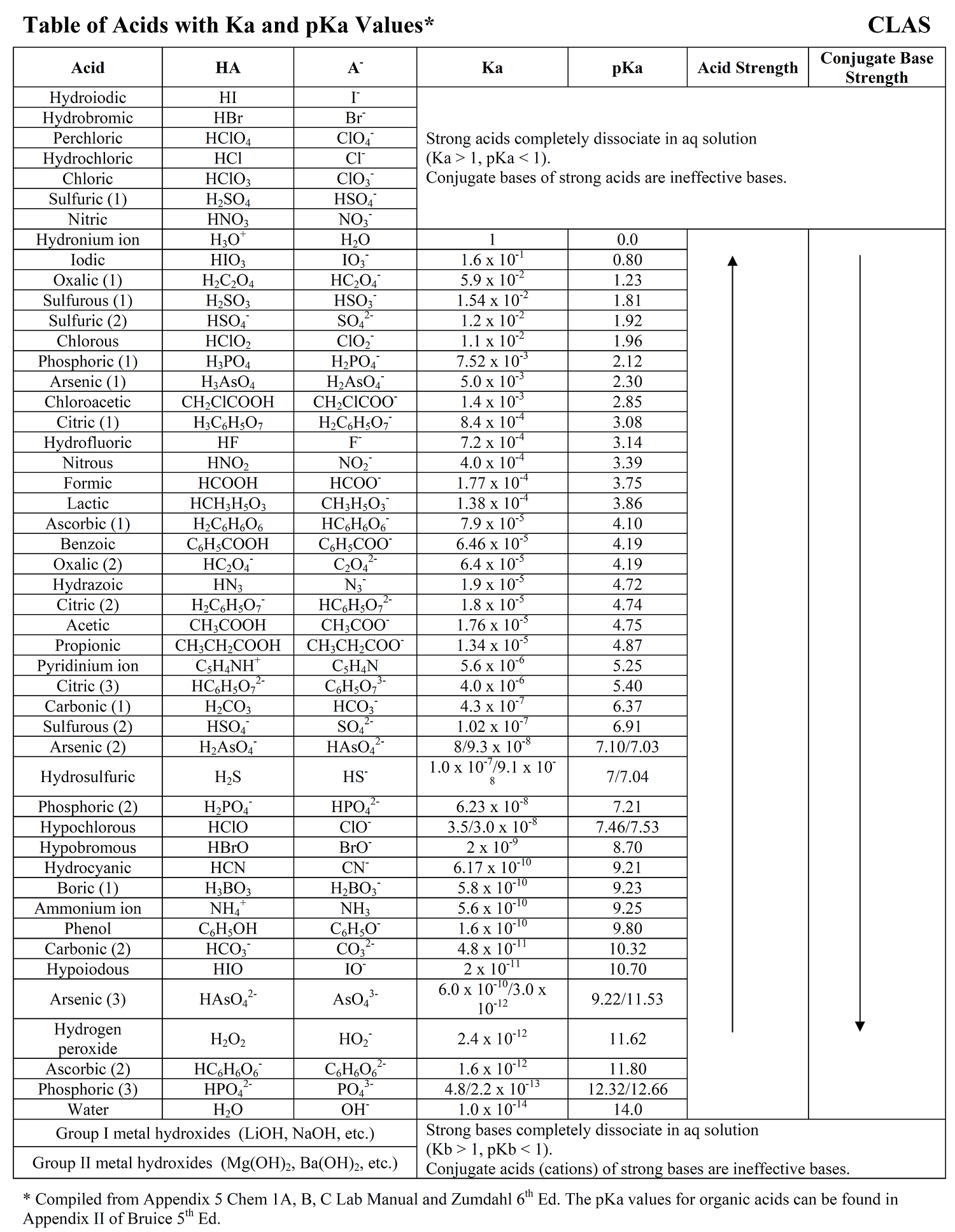
Ka Values And Acids
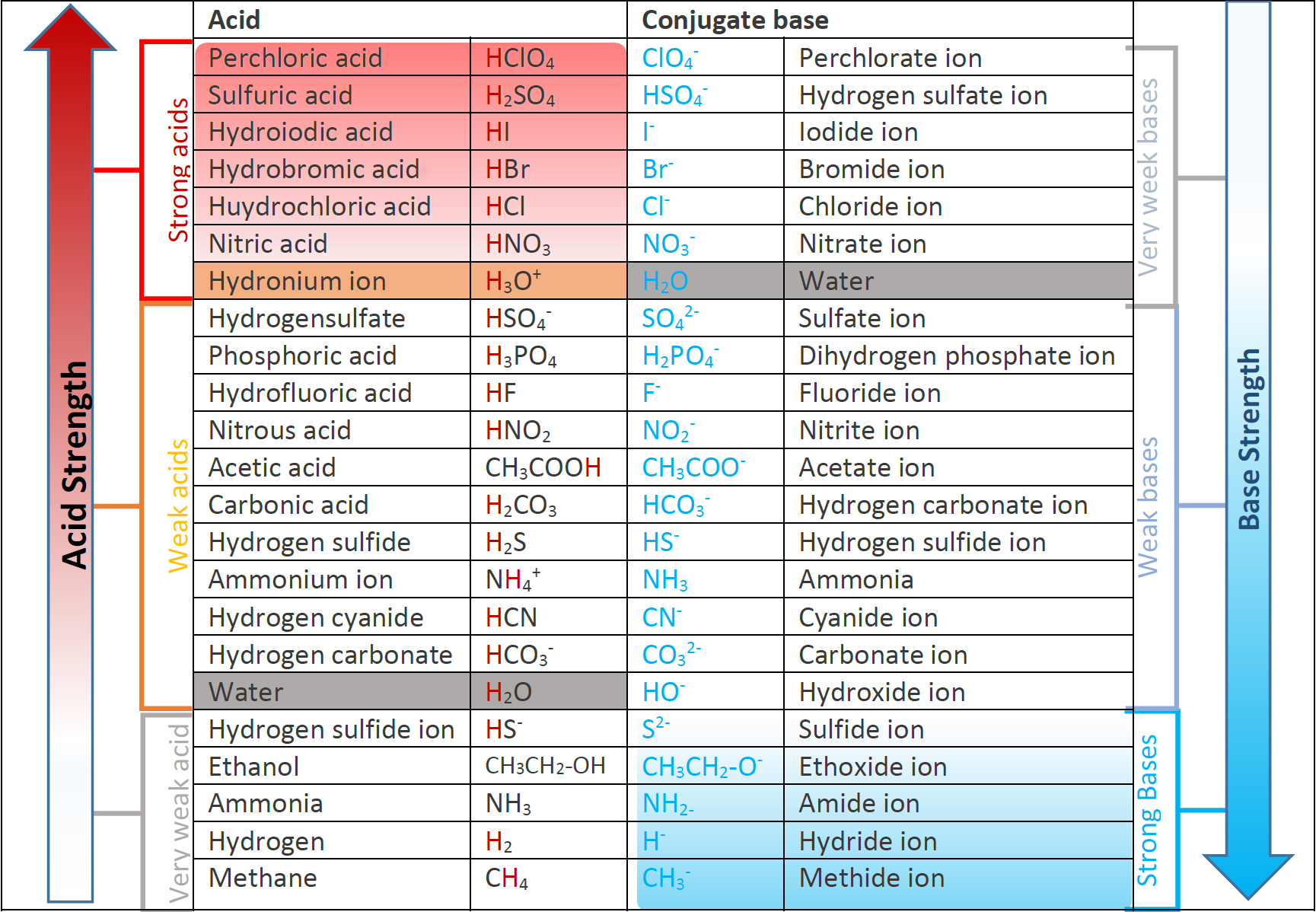
6.3 Strength of acids and bases Chemistry LibreTexts
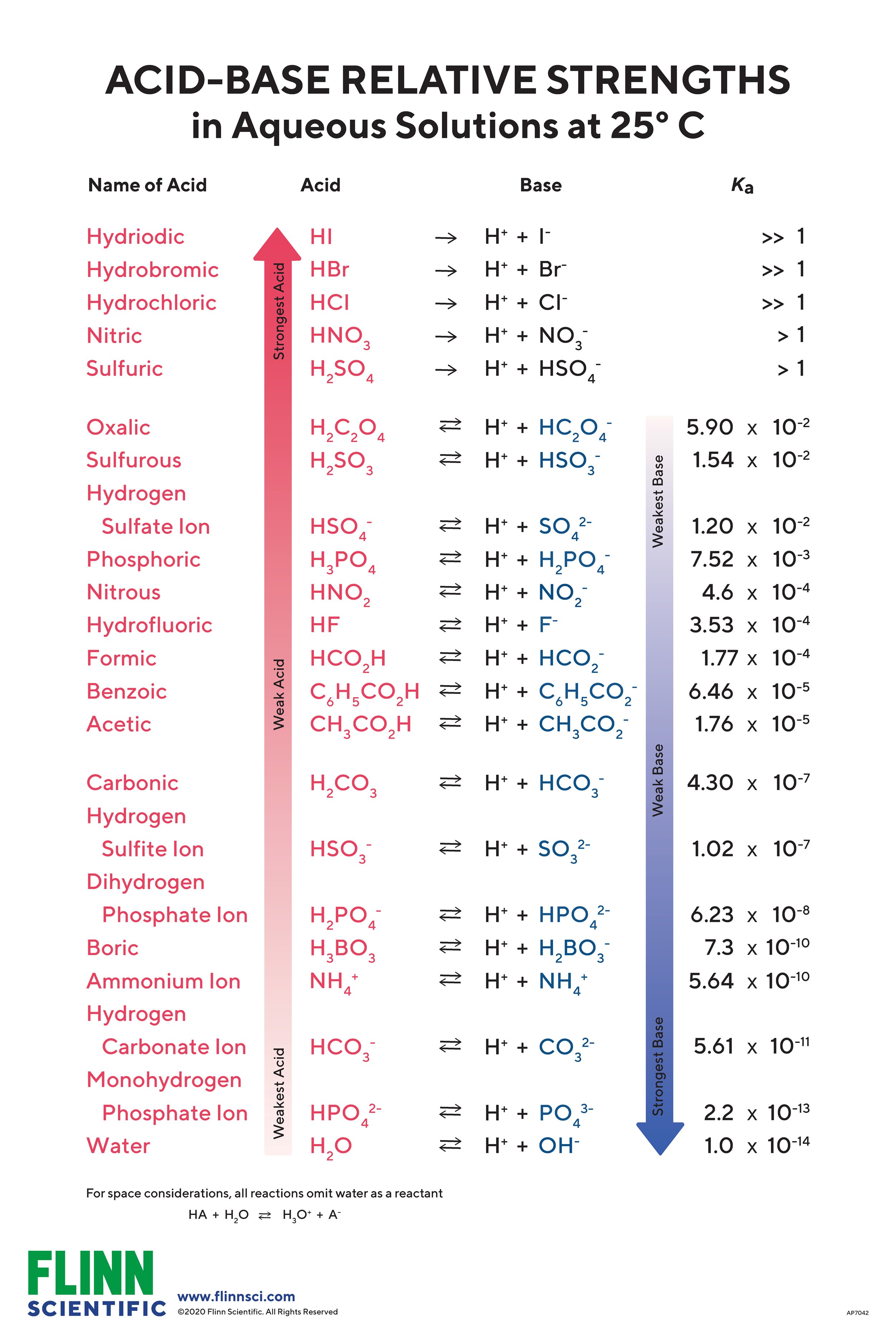
AcidBase Strength Chart
Chart Or Notebook Size Available.
Web Use This Acids And Bases Chart To Find The Relative Strength Of The Most Common Acids And Bases.
By Andy Brunning September 28, 2016.
Web What’s The Diference Between Acid Strength And Concentration?
Related Post:
