Atomic Number Drawing
Atomic Number Drawing - What element is represented by the diagram? • use the periodic table to draw atomic diagrams. Memorization over the winter break i started it off by having the students memorize the first 20 elements (h through ca), in their correct order — by atomic number — over their winter break. Web simple rules to guide you to draw atomic structures! The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. Following hydrogen is the noble gas helium, which has an atomic number of 2. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. Web the atomic number is the number of protons. Since they memorized the elements in order, they should be able to figure this out on their own — but they could also look it up quickly on the periodic table, or look at the element symbol where the atomic number is sometimes written on the lower left. Web an element is defined by the number of protons in its atoms, known as its atomic number. Atomic diagrams (using the periodic table) n atomic number (z) = number of protons. Label them with their charge. Hydrogen, at the upper left of the table, has an atomic number of 1. Counting protons, electrons, and neutrons science >. Web 118 elements and their symbols and atomic numbers. Counting protons, electrons, and neutrons science >. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. Web an element is defined by the number of protons in its atoms, known as its atomic number. Web is to teach them. Web the symbol for an atom can be written to show its mass number at the top, and its atomic number at the bottom. Memorization over the winter break i started it off by having the students memorize the first 20 elements (h through ca), in their correct order — by atomic number — over their winter break. The sum. Draw two electrons in the first energy level and label them with their charge. Web atoms of each element contain a characteristic number of protons. This implies that the nucleus of the sodium atom contains 11 protons and is surrounded by a total. The helium atom contains two protons and two electrons. Since they memorized the elements in order, they. Web atoms of each element contain a characteristic number of protons. Interactive periodic table showing names, electrons, and oxidation states. Web the electron configuration and the orbital diagram are: Beginning with your selected element, determine the atomic number. Atomic weight calculation the mole and avogadro's number atomic number, mass number, and isotopes worked example: The upper right side shows the number of electrons in a neutral atom. Counting protons, electrons, and neutrons science >. Web is to teach them how to draw basic models of atoms. For example, potassium has 19 electrons draw a small circle and write the symbol in the centre. If you want (or need) to draw a model of an. Draw six neutrons in the nucleus of the atom. Memorization over the winter break i started it off by having the students memorize the first 20 elements (h through ca), in their correct order — by atomic number — over their winter break. Web the atomic number is the number of protons. Atomic weight calculation the mole and avogadro's number. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. Web the atomic number tells you how many electrons to draw in total. The helium atom contains two protons and two electrons. The periodic table organizes the elements by atomic. Next on the table is helium, whose atoms have two protons in the nucleus. Web introduction to chemistry preparing to study chemistry elements and atoms average atomic mass worked example: Then play a game to test your ideas! Each element, when electrically neutral, has a number of electrons equal to its atomic number. Memorization over the winter break i started. Every hydrogen atom has one proton in its nucleus. Visualize trends, 3d orbitals, isotopes, and mix. Draw five protons in the nucleus of the atom. Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. The first electron has the same four quantum numbers as the hydrogen atom electron. Web introduction to chemistry preparing to study chemistry elements and atoms average atomic mass worked example: Visualize trends, 3d orbitals, isotopes, and mix. Draw five protons in the nucleus of the atom. The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). Draw two electrons in the first energy level and label them with their charge. Draw three electrons in the second energy level and label them with their charge. Next on the table is helium, whose atoms have two protons in the nucleus. To calculate the numbers of in an atom, use its atomic number and mass number: The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. Web an element is defined by the number of protons in its atoms, known as its atomic number. In contrast, the number of neutrons for a given element can vary. Each element, when electrically neutral, has a number of electrons equal to its atomic number. Web the number of protons in the nucleus is called the atomic number (z) and is the property that defines an atom’s elemental identity. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. Web the electron configuration and the orbital diagram are:/periodic-table-of-the-elements-2017--illustration-769723031-5ac10eb6a9d4f9003769784d.jpg)
Element List Atomic Number, Element Name and Symbol
The Atom Presentation Chemistry

Atomic Structure, Atomic Mass, Atomic Number ⋆
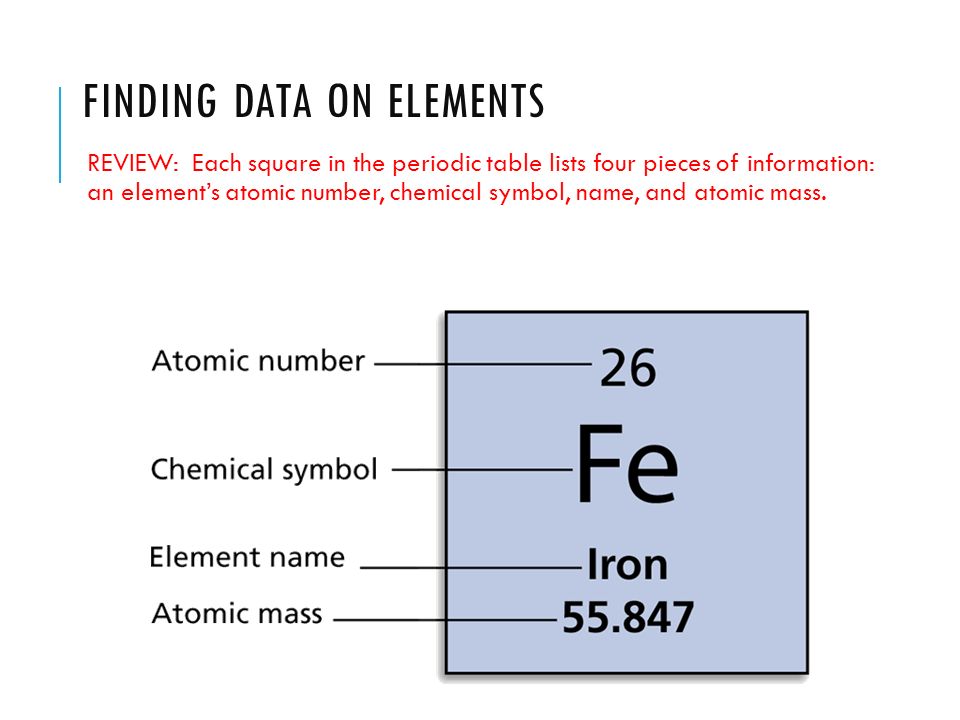
How To Find Element Atomic Number, Element Name & Symbol

Atomic structure Learning Lab
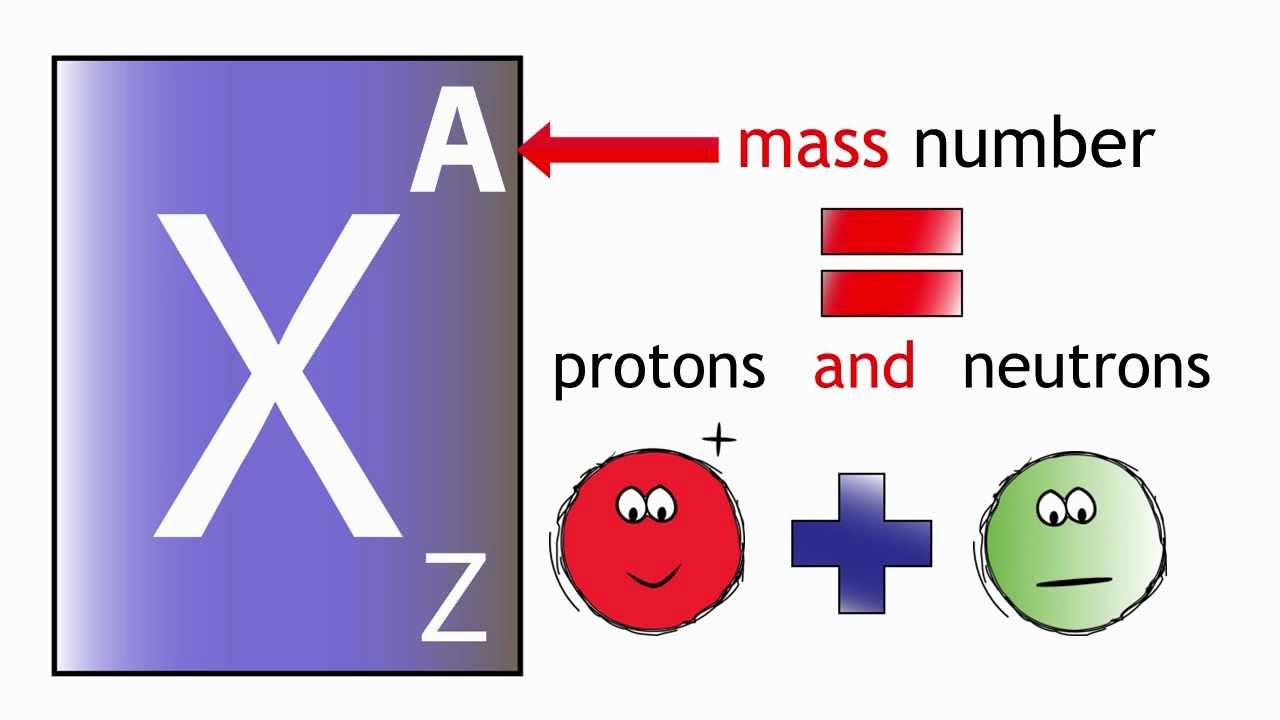
Atomic Number Examples Archives Dynamic Periodic Table of Elements
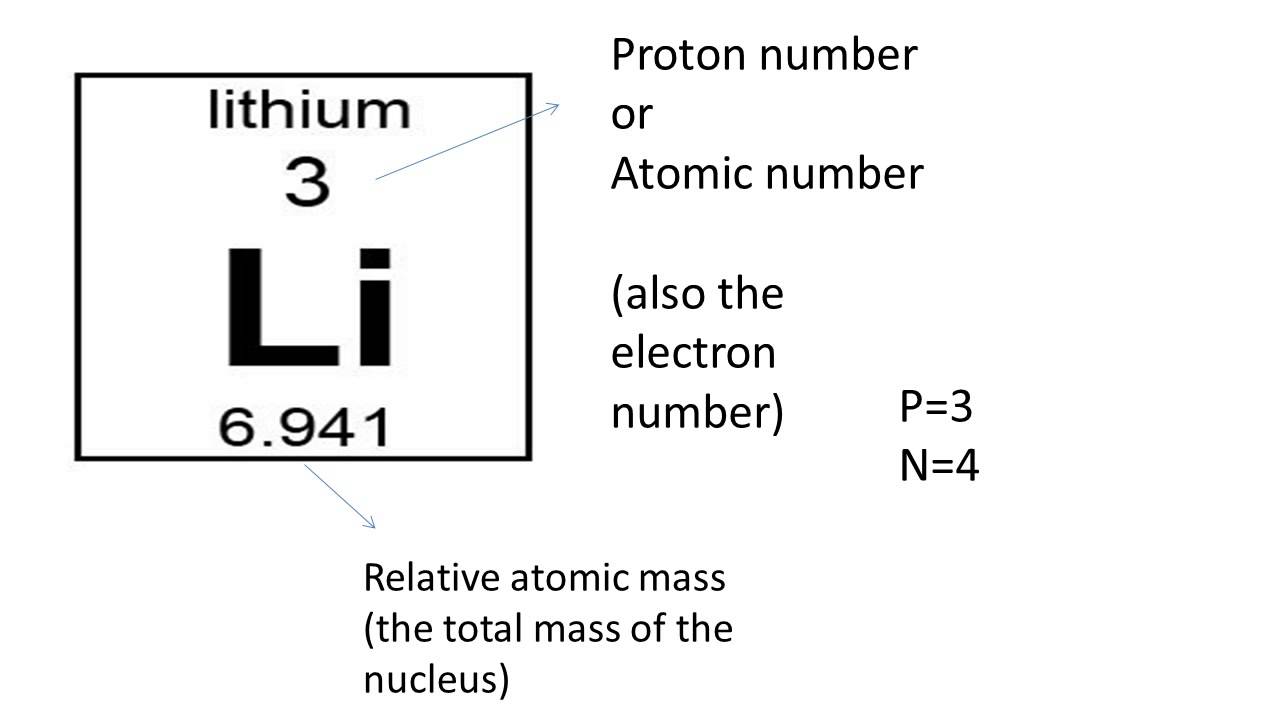
How to draw an atom YouTube
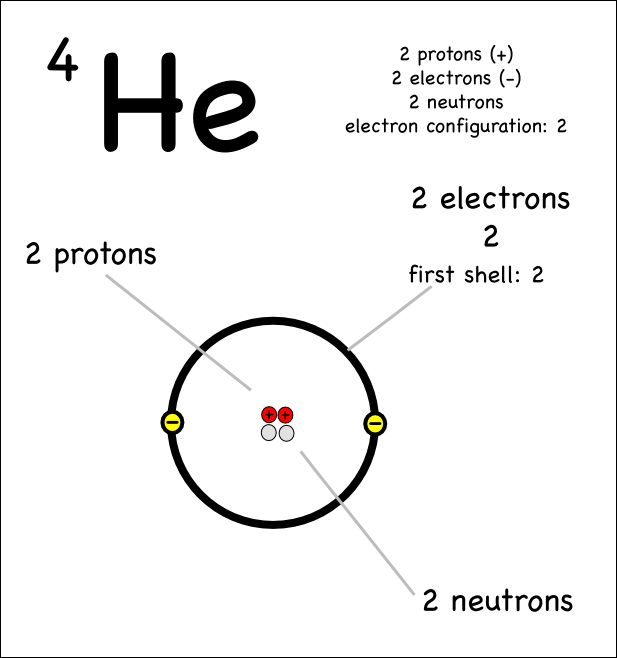
About Atomic Structure
/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)
Element List Atomic Number, Element Name and Symbol
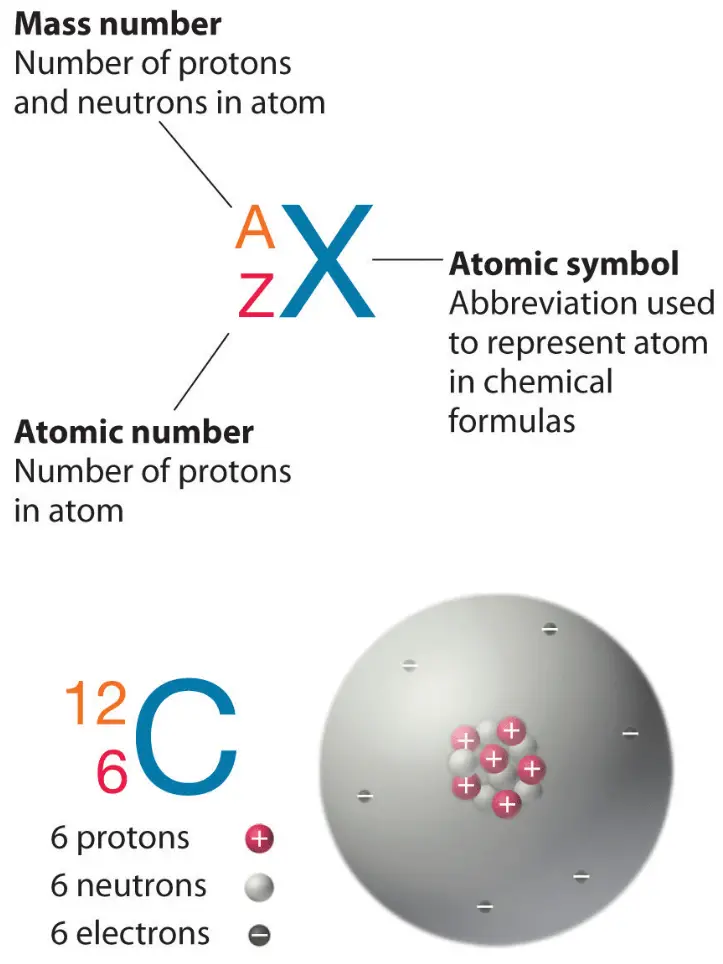
Atomic Mass Number Definition & Characteristics
Following Hydrogen Is The Noble Gas Helium, Which Has An Atomic Number Of 2.
Memorization Over The Winter Break I Started It Off By Having The Students Memorize The First 20 Elements (H Through Ca), In Their Correct Order — By Atomic Number — Over Their Winter Break.
Web Atoms Of Each Element Contain A Characteristic Number Of Protons.
The Number Of Protons In An Atom Is Called The Atomic Number.
Related Post:
.PNG)