Chart Of Chemical Reactions
Chart Of Chemical Reactions - Synthesis reaction, decomposition reaction, single replacement reaction (single displacement reaction), and double replacement reaction (double displacement reaction). Web most chemical reactions can be classified into one or more of five basic types: Scientists classify them based on what happens when going from reactants to products. Web however, when you combine baking soda (nahco 3) and vinegar (ch 3 cooh), the two chemical undergo a chemical reaction that produces sodium acetate (nac 2 h 3 o 2 ), water (h 2 o), and carbon dioxide (co 2 ). What is a chemical reaction? Web many chemical reactions can be classified into one of five basic types. Most of them react with atmospheric oxygen to form metal oxides. The 5 primary types of chemical reactions are: Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and. Web this module will provide an introduction to three of the most prevalent types of chemical reactions: This is helpful in predicting the reactivity of reagents and the products formed from the reactions. Web six common types of chemical reactions are: Browse videos, articles, and exercises by topic. You can think of the reaction as swapping the cations or the anions, but not swapping both since you would end up with the same substances you started with.. The reaction is given below. Web however, when you combine baking soda (nahco 3) and vinegar (ch 3 cooh), the two chemical undergo a chemical reaction that produces sodium acetate (nac 2 h 3 o 2 ), water (h 2 o), and carbon dioxide (co 2 ). Essentially, it involves the rearrangement of atoms. Browse videos, articles, and exercises by. Browse videos, articles, and exercises by topic. Web the four main types of chemical reactions are synthesis, decomposition, single displacement, and double displacement reactions. Web a chemical change, often called a chemical reaction, occurs when substances transform into new and distinct substances. Web a chart of the reactivity series of common metals is provided below. Web 500 possible mastery points. We also discuss what is a combustion reaction, precipitation reaction, and acid base reaction. Unlock the world of chemical reactions! Web a + b − + c + d − → a + d − + c + b −. Web most chemical reactions can be classified into one or more of five basic types: Web a chemical reaction is. Web however, when you combine baking soda (nahco 3) and vinegar (ch 3 cooh), the two chemical undergo a chemical reaction that produces sodium acetate (nac 2 h 3 o 2 ), water (h 2 o), and carbon dioxide (co 2 ). Chemical reactions occur everywhere in the world around you, not just in a chemistry lab. Web most chemical. Get ready to decode the language of chemistry, using symbols and equations to gain a deeper understanding of how substances interact and change at the atomic level. This unit is part of the chemistry library. Web a + b − + c + d − → a + d − + c + b −. Web a chemical reaction is. Unlock the world of chemical reactions! Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and. Precipitation reactions and solubility rules. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as. Web a chart of the reactivity series of common metals is provided below. To keep track of electrons in chemical reactions, oxidation states are assigned to atoms in compounds. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as. This unit is part of the chemistry library. This is helpful in predicting the reactivity of reagents and the products formed from the reactions. A chemical. Web six common types of chemical reactions are: Web this module will provide an introduction to three of the most prevalent types of chemical reactions: Web most chemical reactions can be classified into one or more of five basic types: Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories.. Chemical reactions tend to involve the motion of electrons, leading to the formation and breaking of chemical bonds. Metals tend to readily lose electrons and form cations. Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. This unit is part of the chemistry library. A chemical reaction is a. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Precipitation reactions and solubility rules. Web 500 possible mastery points. Unlock the world of chemical reactions! C6h12o6 + 6o2 → 6co2 + 6h2o + energy. Web examples of chemical reactions in everyday life include photosynthesis, rust, baking, digestion, combustion, chemical batteries, fermentation, and washing with soap and water. Web a chemical reaction is a process generally characterized by a chemical change in which the starting materials (reactants) are different from the products. Web a chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and. A chemical reaction is a process or chemical change that transforms one set of substances (the reactants) into another set of substances (the products). To keep track of electrons in chemical reactions, oxidation states are assigned to atoms in compounds. This unit is part of the chemistry library. Web many chemical reactions can be classified into one of five basic types. Metals tend to readily lose electrons and form cations. A chemical equation represents the total chemical change that occurs in a chemical reaction using symbols and chemical formulas for the substances involved.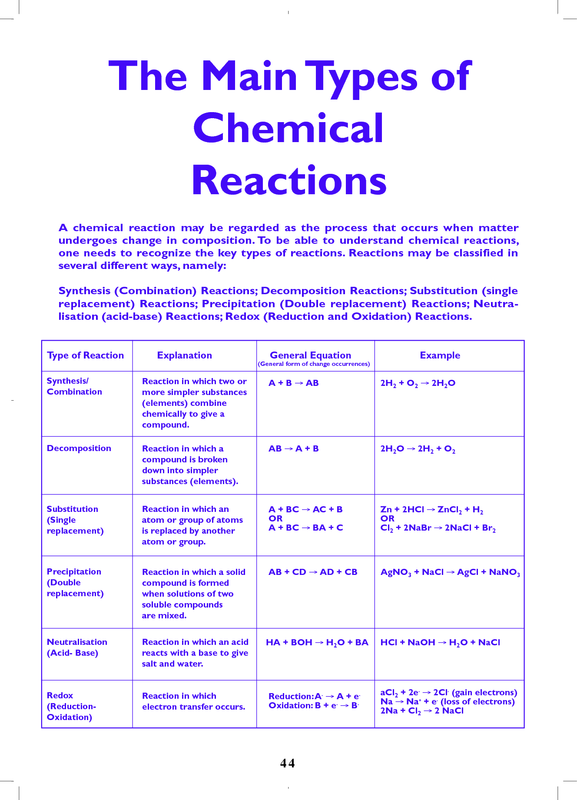
C3.1 Chemical Reactions
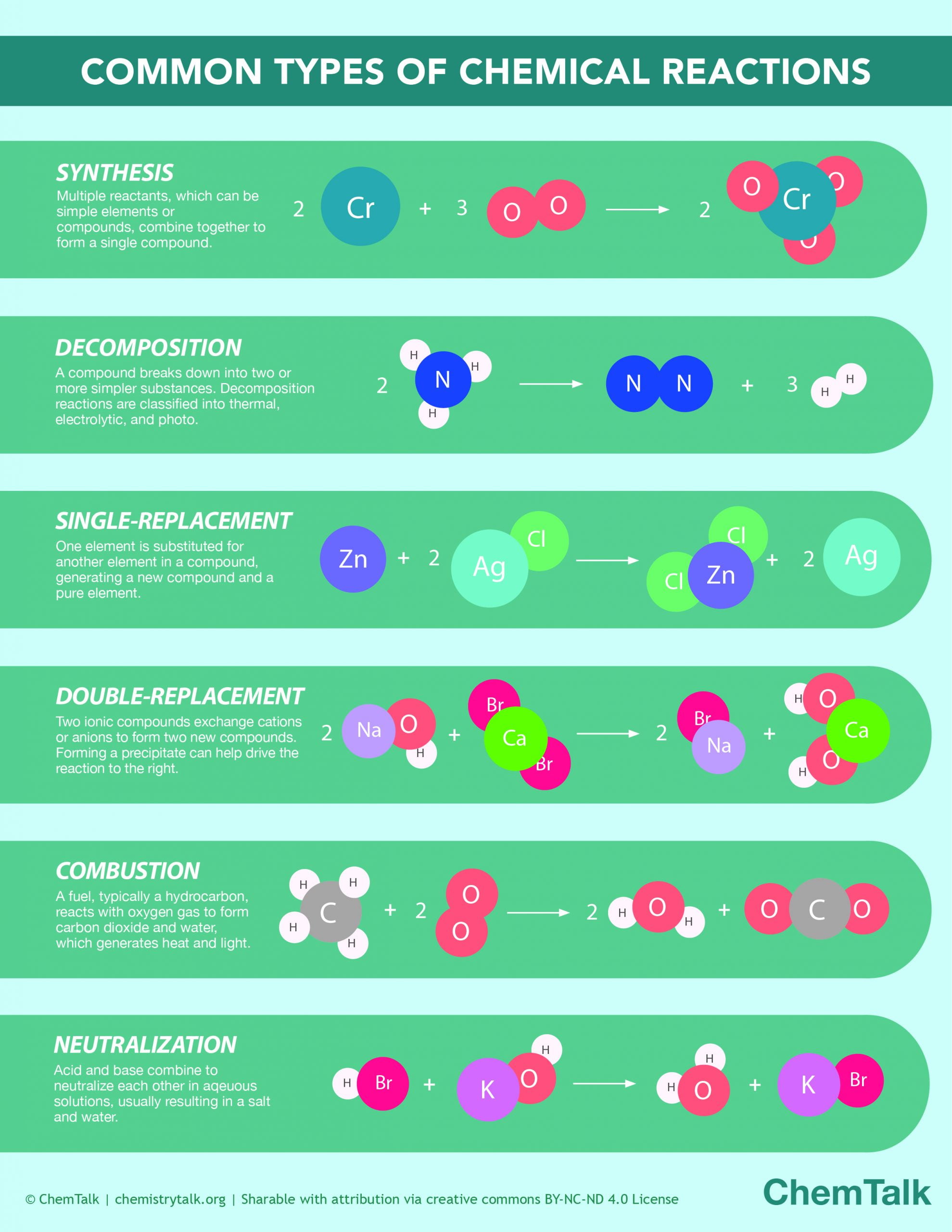
Chemical Reactions Infographic ChemTalk
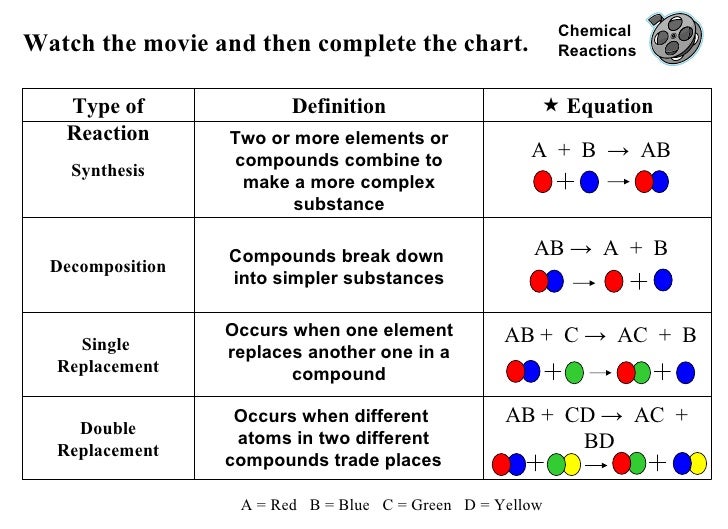
Types of chemical reactions

Explain Four Different Types of Chemical Reaction With Suitable Examples

Types of Chemical Reactions Chemical reactions, Chemistry basics
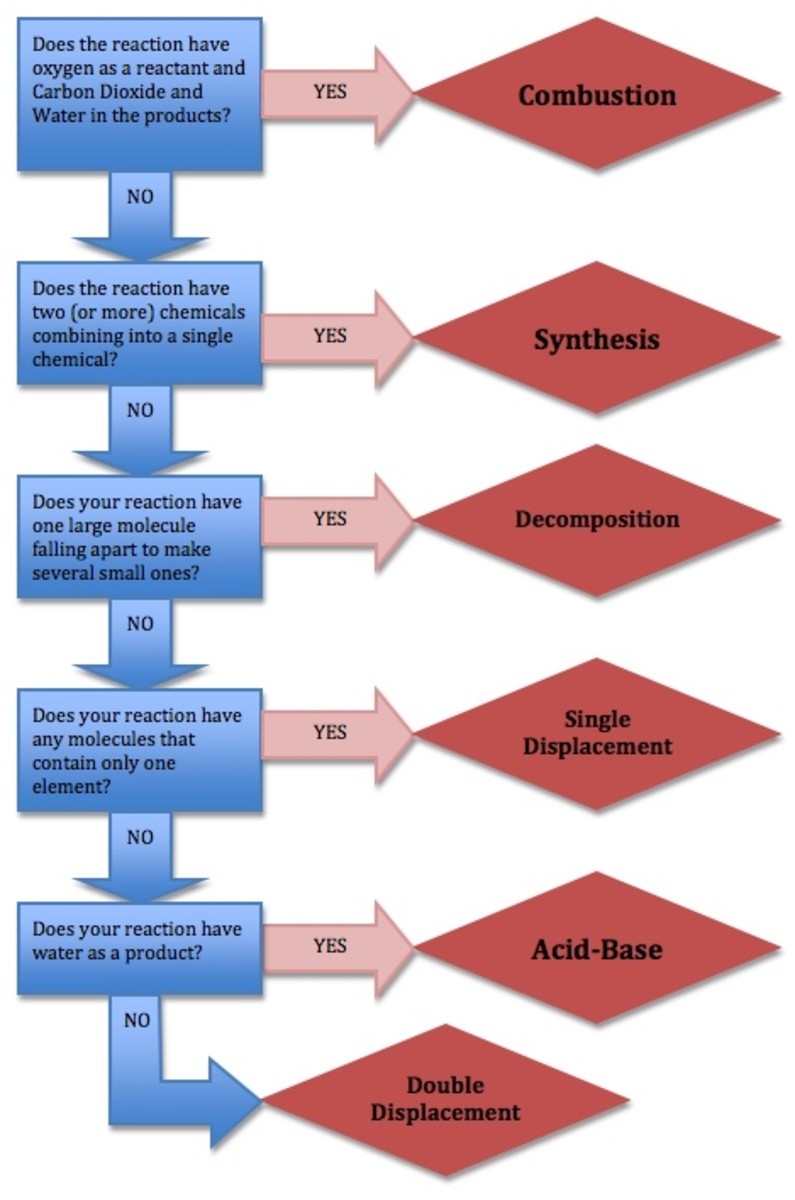
The 6 Types of Chemical Reactions Owlcation
Chemical Reactions — The Wonder of Science

Chem Awareness Chemical Reactions
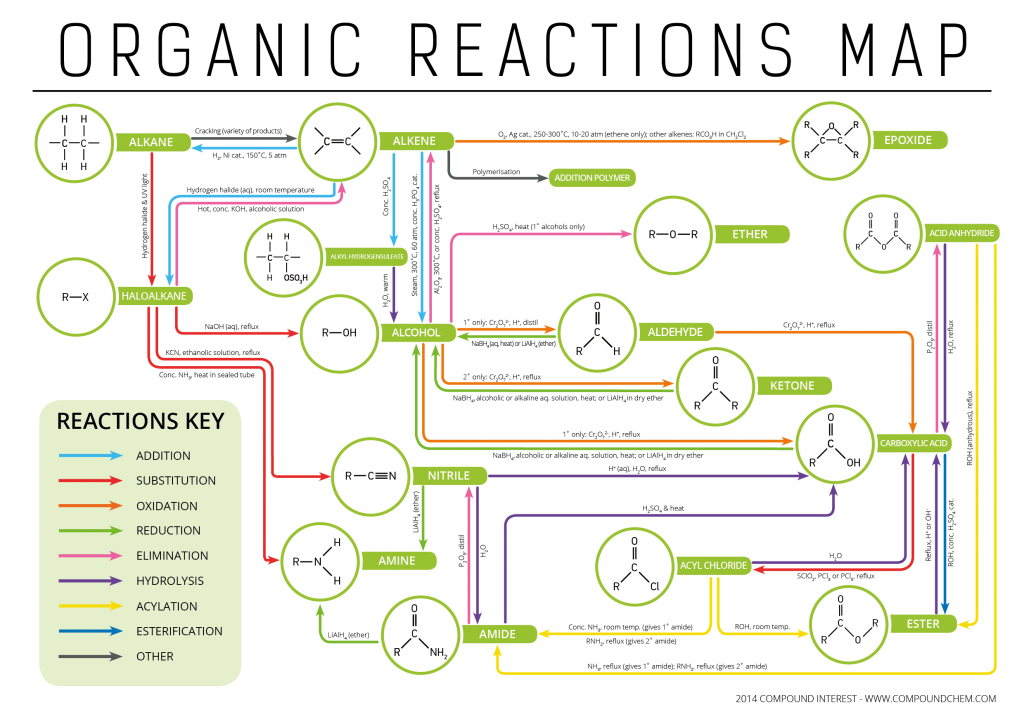
Types of Organic Reactions Functional Groups Interconversion
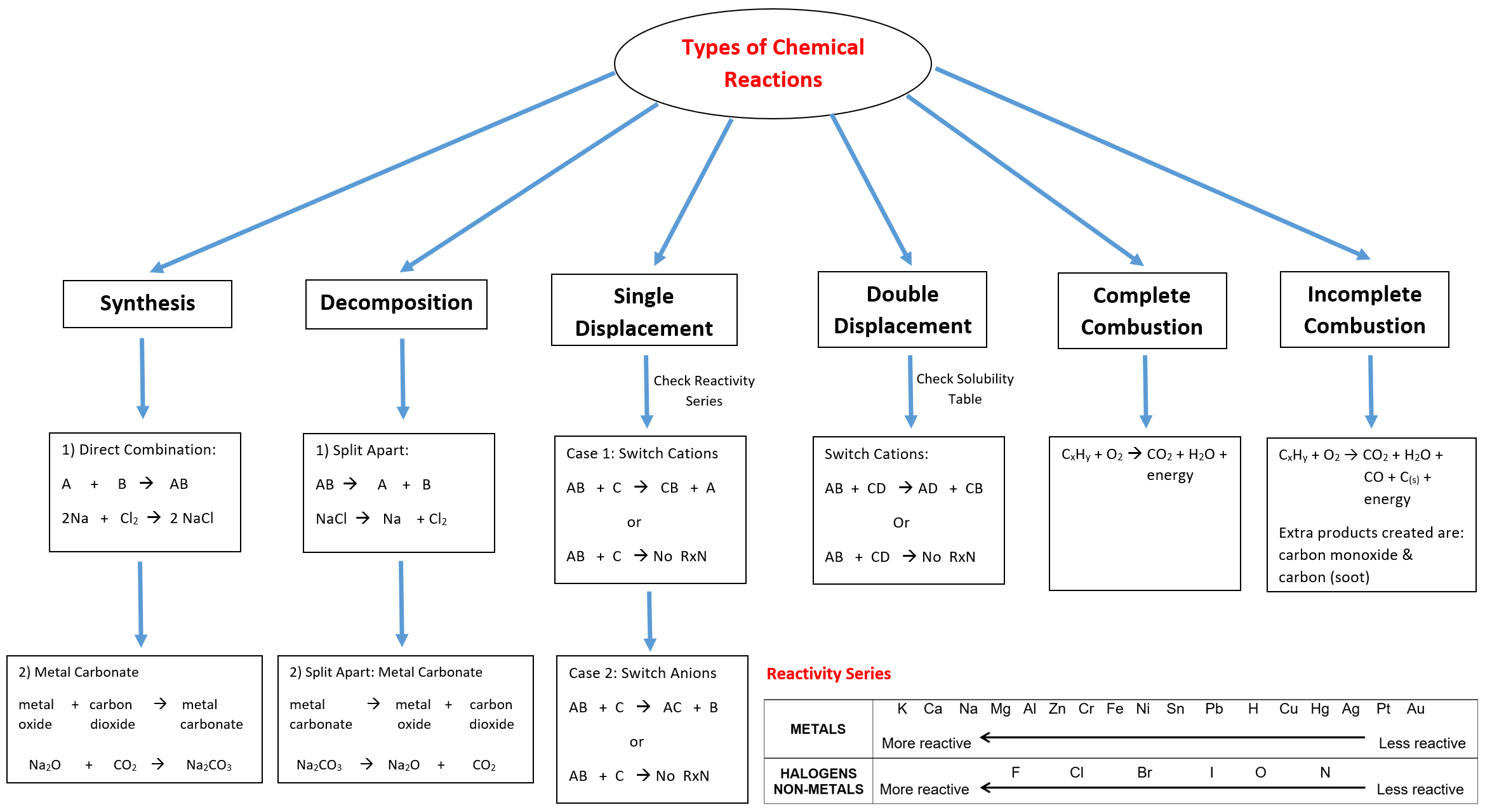
Chemistry Ch.6&7 Chemical Reactions & Acids/Bases Mr.Panchbhaya's
Web The Four Main Types Of Chemical Reactions Are Synthesis, Decomposition, Single Displacement, And Double Displacement Reactions.
Some Reactions Will Fit Into More Than One Category.
Chemical Reactions Occur Everywhere In The World Around You, Not Just In A Chemistry Lab.
Web This Module Will Provide An Introduction To Three Of The Most Prevalent Types Of Chemical Reactions:
Related Post:
