Chemical Reactions Chart
Chemical Reactions Chart - A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. Ch 4 + 2o 2 → co 2 + 2h 2 o. Web the 5 primary types of chemical reactions are: The starting substances are called the reactants, and the new substances that form are called the products. In real life, your reaction flask might look something like the picture below. Not all changes involving matter are chemical reactions. If not, write nr nr. Web most chemical reactions can be classified into one or more of five basic types: Most of them react with atmospheric oxygen to form metal oxides. Web common types of chemical reactions. Cooking measurement converter cooking ingredient converter. Reactions are classified into thermal, electrolytic,. If not, write nr nr. Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. Web a chart of the reactivity series of common metals is provided below. Web interactive periodic table showing names, electrons, and oxidation states. Most of them react with atmospheric oxygen to form metal oxides. A chemical reaction is a process or chemical change that transforms one set of substances (the reactants) into another set of substances (the products). The decomposition of calcium carbonate: Web the chemical reactivity worksheet (crw) is a free software. Multiple reactants, which can be. Web 500 possible mastery points. A reaction in which two or more reactants combine to form a single product is known as a combination reaction. The decomposition of calcium carbonate: In real life, your reaction flask might look something like the picture below. Caco 3 → cao + co 2. Analyzing the reactants and products of a given reaction will allow you to place it into one of these categories. Metals tend to readily lose electrons and form cations. Just like running, it takes practice and dedication. Reactions are classified into thermal, electrolytic,. In real life, your reaction flask might look something like the picture below. Web common types of chemical reactions. Metals tend to readily lose electrons and form cations. Combining aqueous solutions of lead (ii) nitrate and potassium iodide results in the formation of insoluble lead (ii) iodide, a yellow solid. A chemical reaction is in which the bonds are broken. A reaction in which two or more reactants combine to form a single product is known as a combination reaction. Ag(s) + hcl(aq) → ag ( s) + hcl (. The starting substances are called the reactants, and the new substances that form are called the products. Anyone can access the reactions highlighted in red. If not, write nr nr. Web a chemical reaction is a process in which one or more substances are converted to one or more different substances. Web chemix is a free online editor for drawing science lab diagrams and school experiment apparatus. Not all changes involving matter are chemical reactions. If the reaction does occur, write the products of the reaction and balance the equation.. Cooking measurement converter cooking ingredient converter. How to recognize a chemical reaction. Some reactions will fit into more than one category. A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. If not, write nr nr. Chemical reactions occur everywhere in the world around you, not just in a chemistry lab. Just like running, it takes practice and dedication. A compound breaks down into two or. Get ready to decode the language of chemistry, using symbols and equations to gain a deeper understanding of how substances interact and change at the atomic level. Web pb (no. Web chemix is a free online editor for drawing science lab diagrams and school experiment apparatus. Web a chart of the reactivity series of common metals is provided below. Unlock the world of chemical reactions! Caco 3 → cao + co 2. How to recognize a chemical reaction. A compound breaks down into two or. A chemical reaction is a chemical change, which means the starting materials are chemically. The decomposition of calcium carbonate: If not, write nr nr. Caco 3 → cao + co 2. Web the 5 primary types of chemical reactions are: How to recognize a chemical reaction. Web the combustion of methane: Visualize trends, 3d orbitals, isotopes, and mix compounds. Web pb (no 3) 2 ( a q) + 2 ki ( a q) → 2 kno 3 ( a q) + pbi 2 ( s) we made a beautiful golden solid from two clear solutions! Metals tend to readily lose electrons and form cations. Web this unit is part of the chemistry library. Browse videos, articles, and exercises by topic. Chemical reactions tend to involve the motion of electrons, leading to the formation and breaking of chemical bonds. Reactions are classified into thermal, electrolytic,. Al(s) + zn(no3)2(aq) → al ( s) + zn ( no 3) 2 ( a q) →.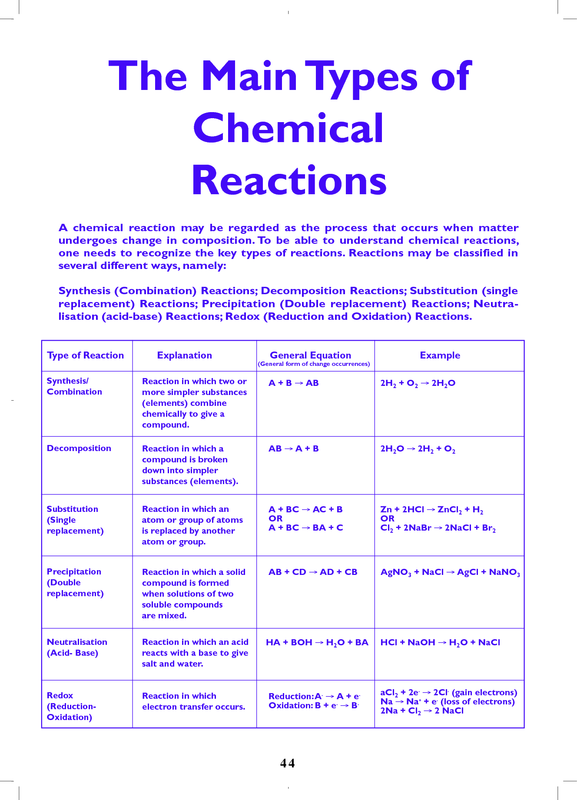
C3.1 Chemical Reactions
:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
Types of Chemical Reactions (With Examples)

Types of Chemical Reactions

Explain Four Different Types of Chemical Reaction With Suitable Examples

Classifying Chemical Reactions Stacy Goldstein Library Formative
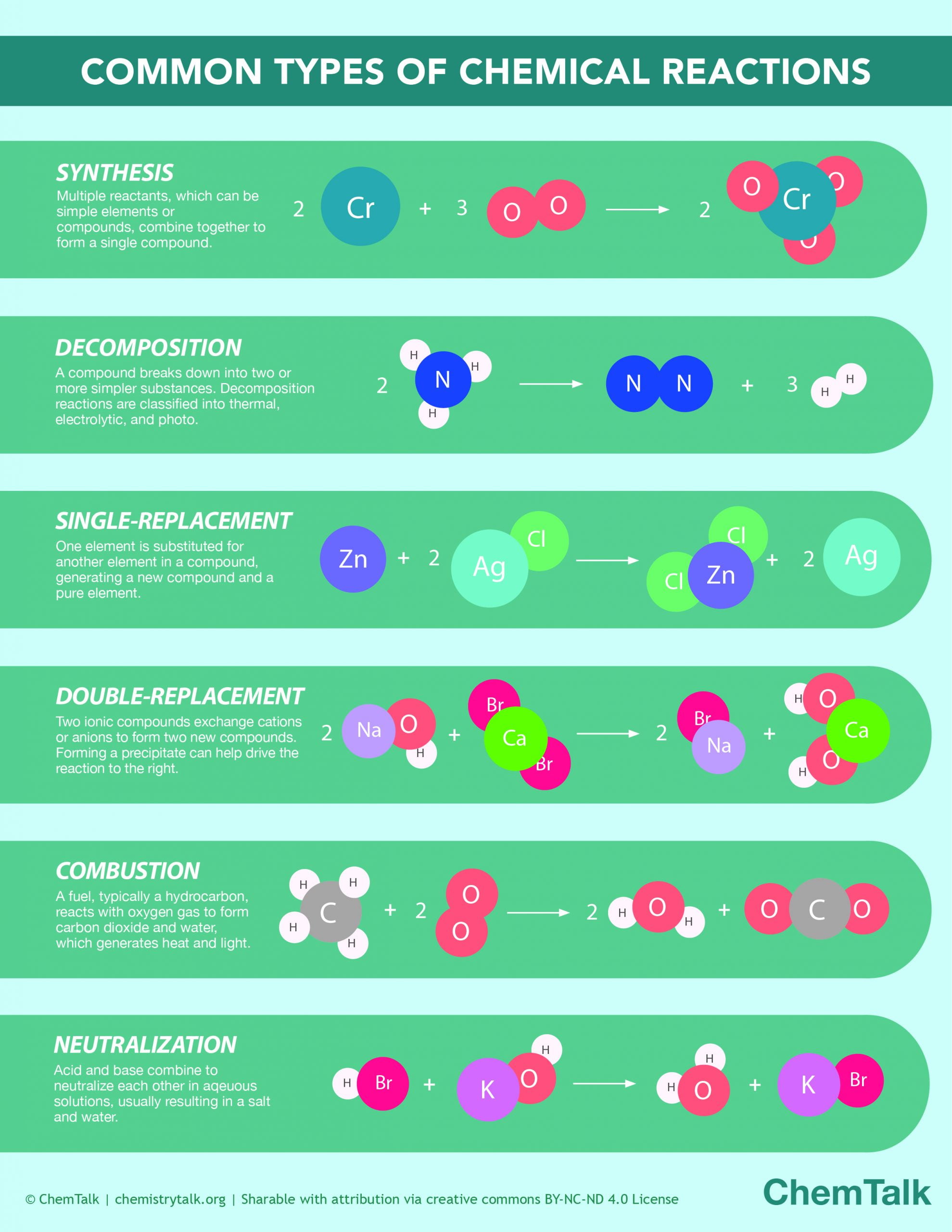
visual presentation of a chemical reaction
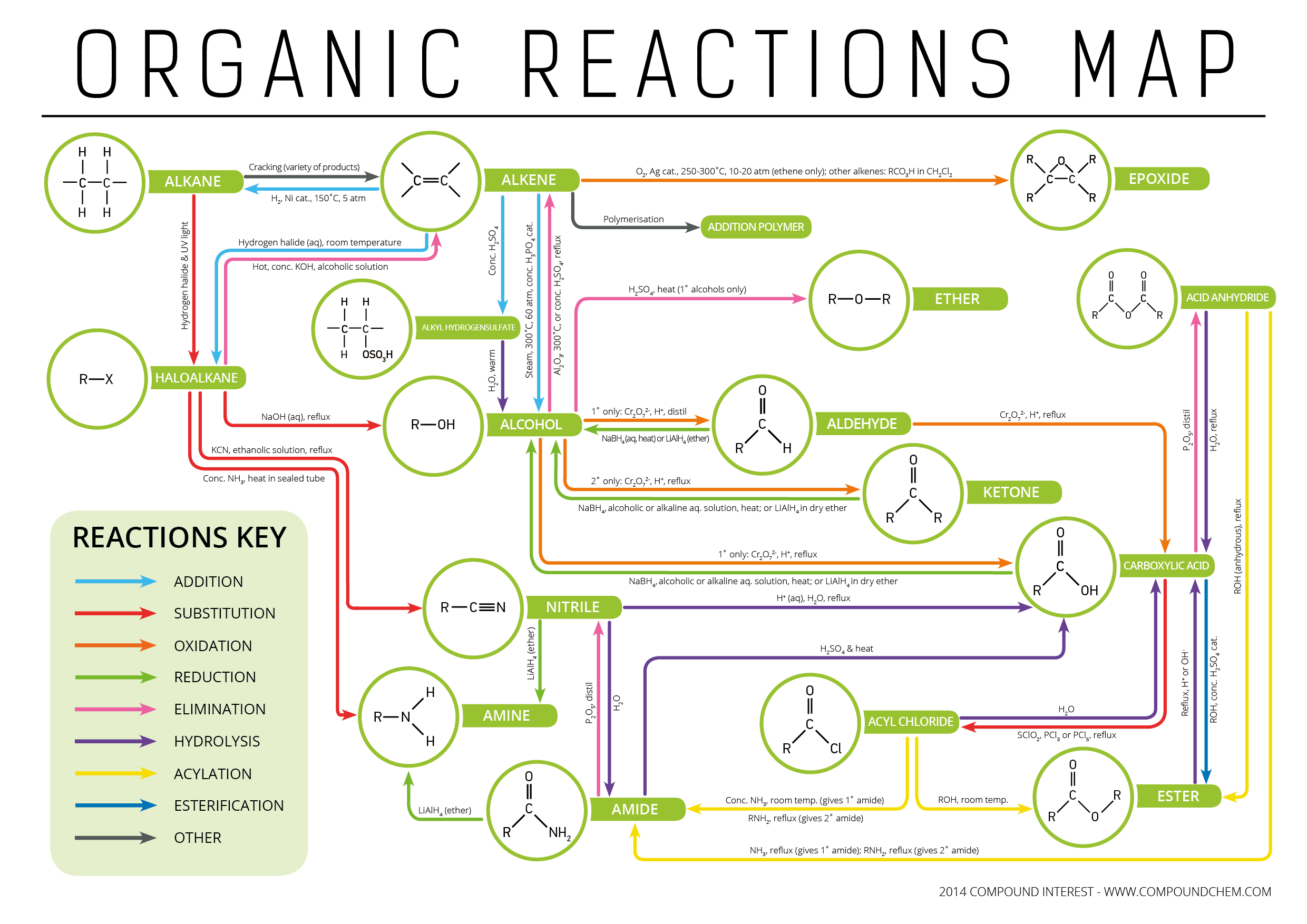
Types of Organic Reactions Functional Groups Interconversion
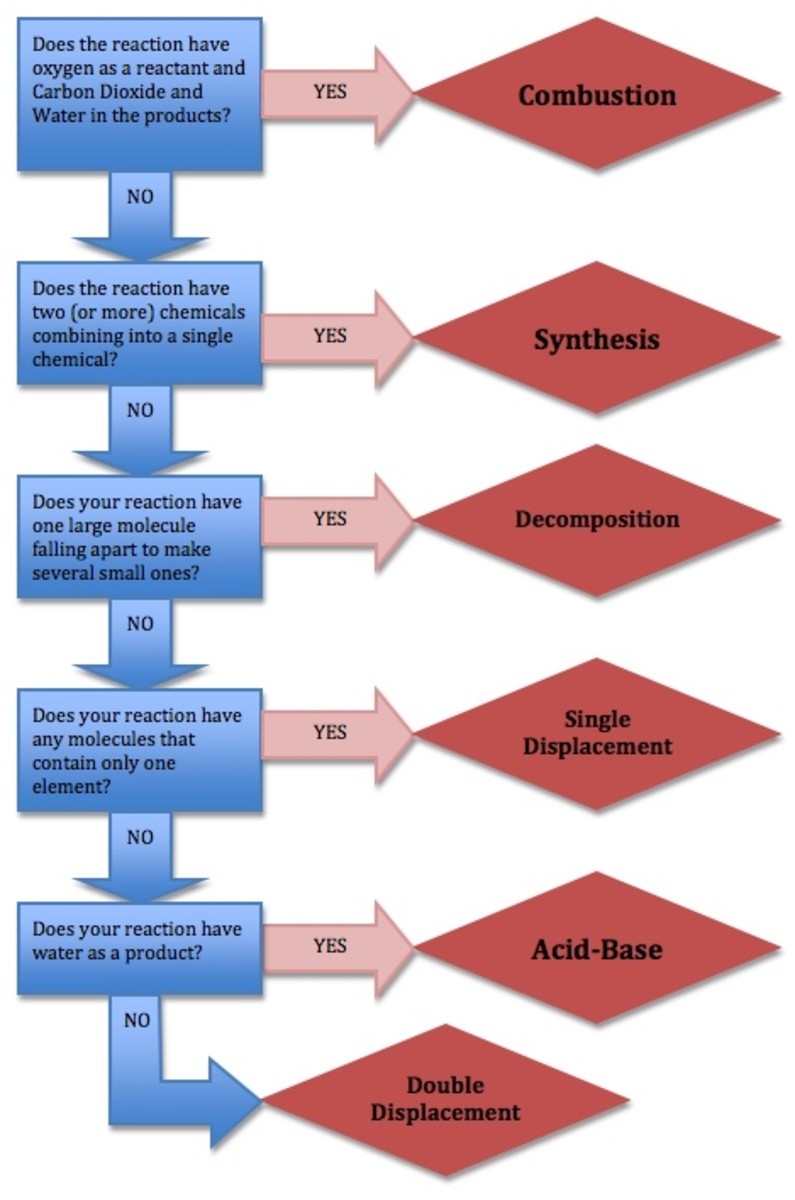
The 6 Types of Chemical Reactions Owlcation

Chem Awareness Chemical Reactions
Chemical Reactions — The Wonder of Science
Multiple Reactants, Which Can Be.
Combining Aqueous Solutions Of Lead (Ii) Nitrate And Potassium Iodide Results In The Formation Of Insoluble Lead (Ii) Iodide, A Yellow Solid.
The Starting Substances Are Called The Reactants, And The New Substances That Form Are Called The Products.
Web The Four Main Types Of Chemical Reactions Are Synthesis, Decomposition, Single Displacement, And Double Displacement Reactions.
Related Post:
