Clinical Trials Phases Flow Chart
Clinical Trials Phases Flow Chart - This video explains what’s involved in the three main phases. Learn what happens during each phase. Knowing the phase of the clinical trial is important because it can give you some idea about how much is known about the treatment being studied. Phase 1 clinical trials are usually the first to involve people, and help doctors learn if a new treatment is safe. Web the four phases of clinical trials. Web for the purposes of management, it is useful to consider explicitly three phases of the trial execution process: Phase 1 trials are the earliest phase trials and phase 3 are later phase trials. Explore the definitions of phase 1, 2, 3 and 4 clinical trials and learn how to find a trial that fits your needs. Clinical trials to test new cancer treatments involve a series of steps, called phases. Who takes part in clinical trials? Web in this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: Tests a new therapy for the first time in a small group of people to determine if it is safe, find the right dose, and learn about side effects. Phase 1 trials are the earliest phase trials and. Web clinical trials are conducted according to a plan, called a protocol, which describes: Web here is a chart about the different phases of clinical trials. Web this article describes the different clinical trial phases in order to inform current and future clinical trial participants, their loved ones, and caregivers about what to expect during a particular phase regardless of. Web in this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: Clinical trials are usually conducted in phases that build on one another. Web this article describes the different clinical trial phases in order to inform current and future clinical trial participants, their loved ones, and caregivers about what. Each phase is designed to answer certain questions. Clinical trials to test new cancer treatments involve a series of steps, called phases. Web clinical trials happen in four different phases: Most go through a multiphase clinical trial. Who takes part in clinical trials? Phase 0, phase i, phase ii, phase iii, and phase iv. Web for the purposes of management, it is useful to consider explicitly three phases of the trial execution process: They may also explore different dosing schedules or new drug combinations. They may have already tested it in laboratory animals. Web this video explains the main phases of clinical trials. When expressed specifically, a clinical trial phase is capitalized both in name and roman numeral, such as phase i clinical trial. Phase i trials are the first time a drug is being used in humans. Involves more people to determine how well the new therapy treats a disease and whether the treatment is safe. Web clinical trials are conducted according. What do the terms placebo, randomization, and blinded mean in clinical trials? Clinical trials to test new cancer treatments involve a series of steps, called phases. This video explains what’s involved in the three main phases. The trial execution phase, comprising those activities (enrollment, measurement etc.) that constitute the major trial activities, and relying heavily on study monitoring and evalua.. Clinical trials to test new cancer treatments involve a series of steps, called phases. Learn what happens during each phase. The trial execution phase, comprising those activities (enrollment, measurement etc.) that constitute the major trial activities, and relying heavily on study monitoring and evalua. The types of patients who may enter the study. Phase 0, phase i, phase ii, phase. How is my safety protected? They may also explore different dosing schedules or new drug combinations. Tests a new therapy for the first time in a small group of people to determine if it is safe, find the right dose, and learn about side effects. Web clinical trials are conducted according to a plan, called a protocol, which describes: What. They contribute significantly to relevant research evidence developed by the nihr to support the nhs in england and other care providers. Each phase is designed to answer certain questions. Phase i trials are the first time a drug is being used in humans. The drug development process will normally proceed through all four phases over many years. Phase 0, phase. Web here is a chart about the different phases of clinical trials. Phase 1 clinical trials are usually the first to involve people, and help doctors learn if a new treatment is safe. Explore the definitions of phase 1, 2, 3 and 4 clinical trials and learn how to find a trial that fits your needs. Web clinical trials are conducted according to a plan, called a protocol, which describes: They may have already tested it in laboratory animals. What are the phases of a clinical trial? Phase 0, phase i, phase ii, phase iii, and phase iv. How is my safety protected? Who takes part in clinical trials? The types of patients who may enter the study. Web for the purposes of management, it is useful to consider explicitly three phases of the trial execution process: Is the new treatment safe? Clinical trials are usually conducted in phases that build on one another. Web this video explains the main phases of clinical trials. Web this article describes the different clinical trial phases in order to inform current and future clinical trial participants, their loved ones, and caregivers about what to expect during a particular phase regardless of the treatment or medical condition being studied. Clinical trials to test new cancer treatments involve a series of steps, called phases.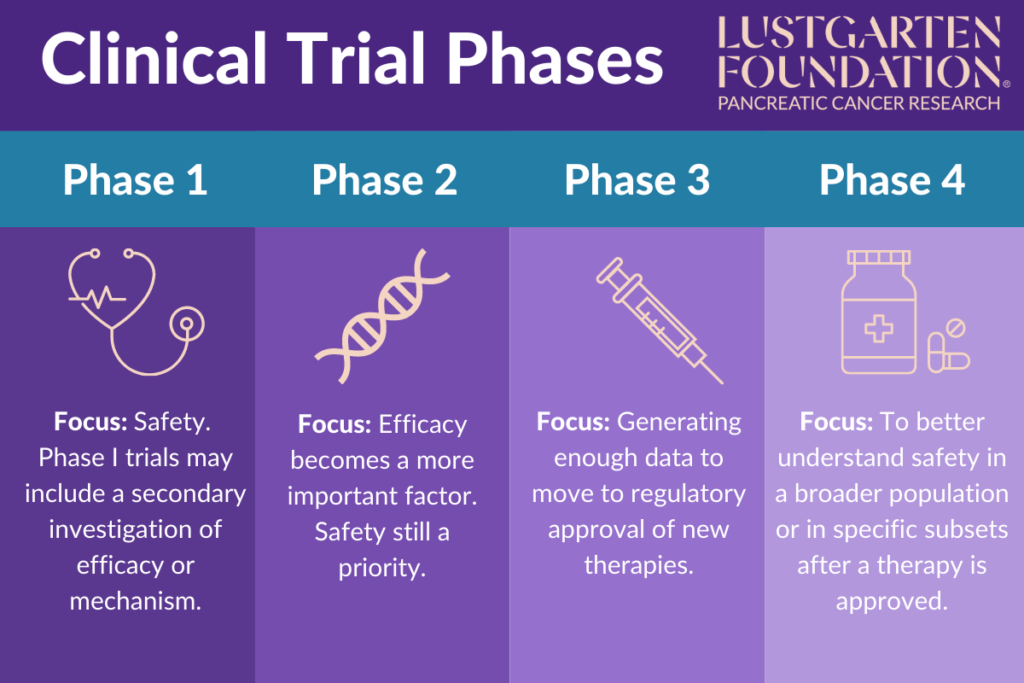
Clinical Trial Phases

Phase 1 clinical trials Health Research Authority
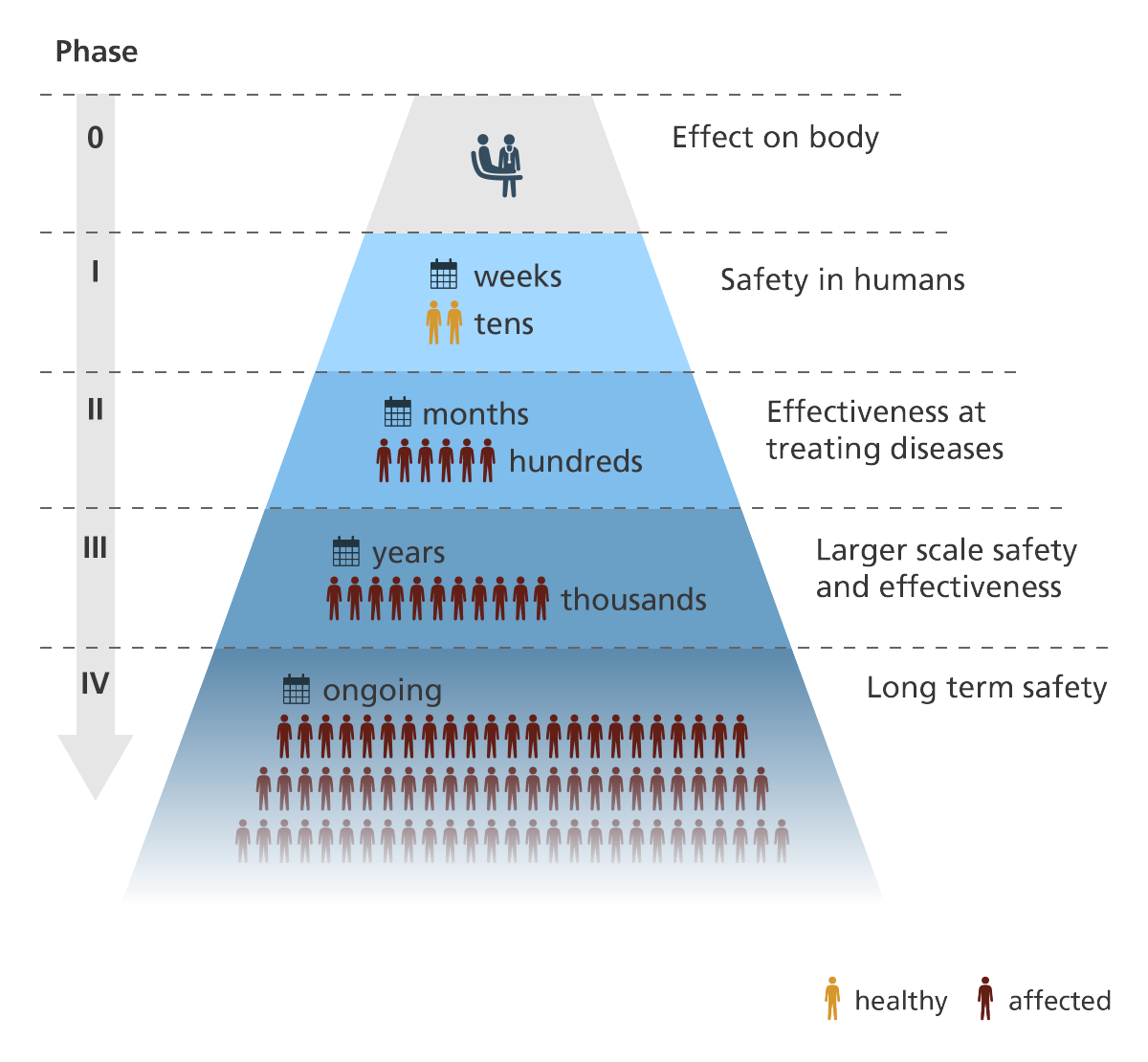
Understanding the Clinical Trial Process MStranslate
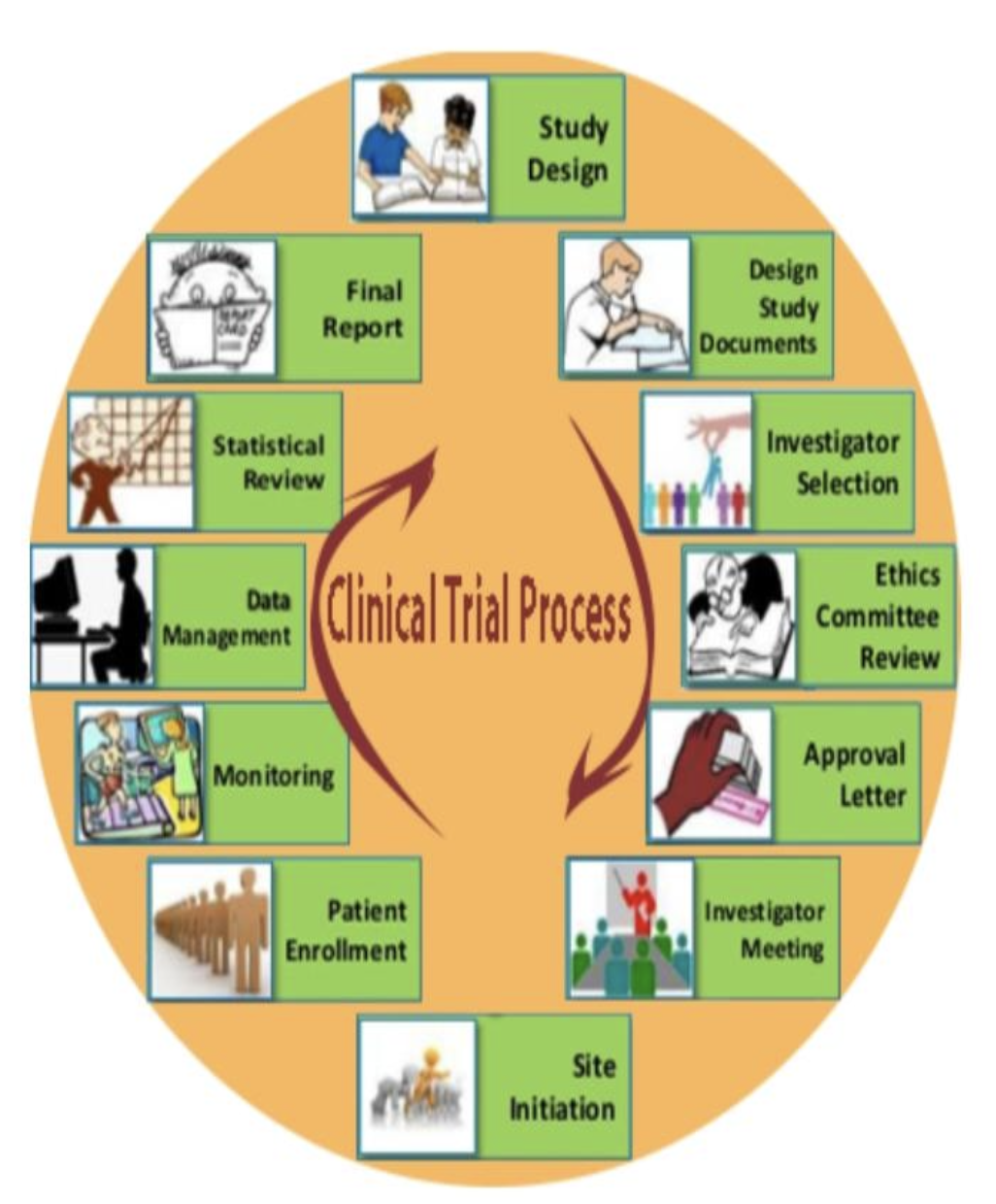
Fundamentals of Clinical Trials Phases of Clinical Trials CCRPS

Clinical trial flowchart. Download Scientific Diagram
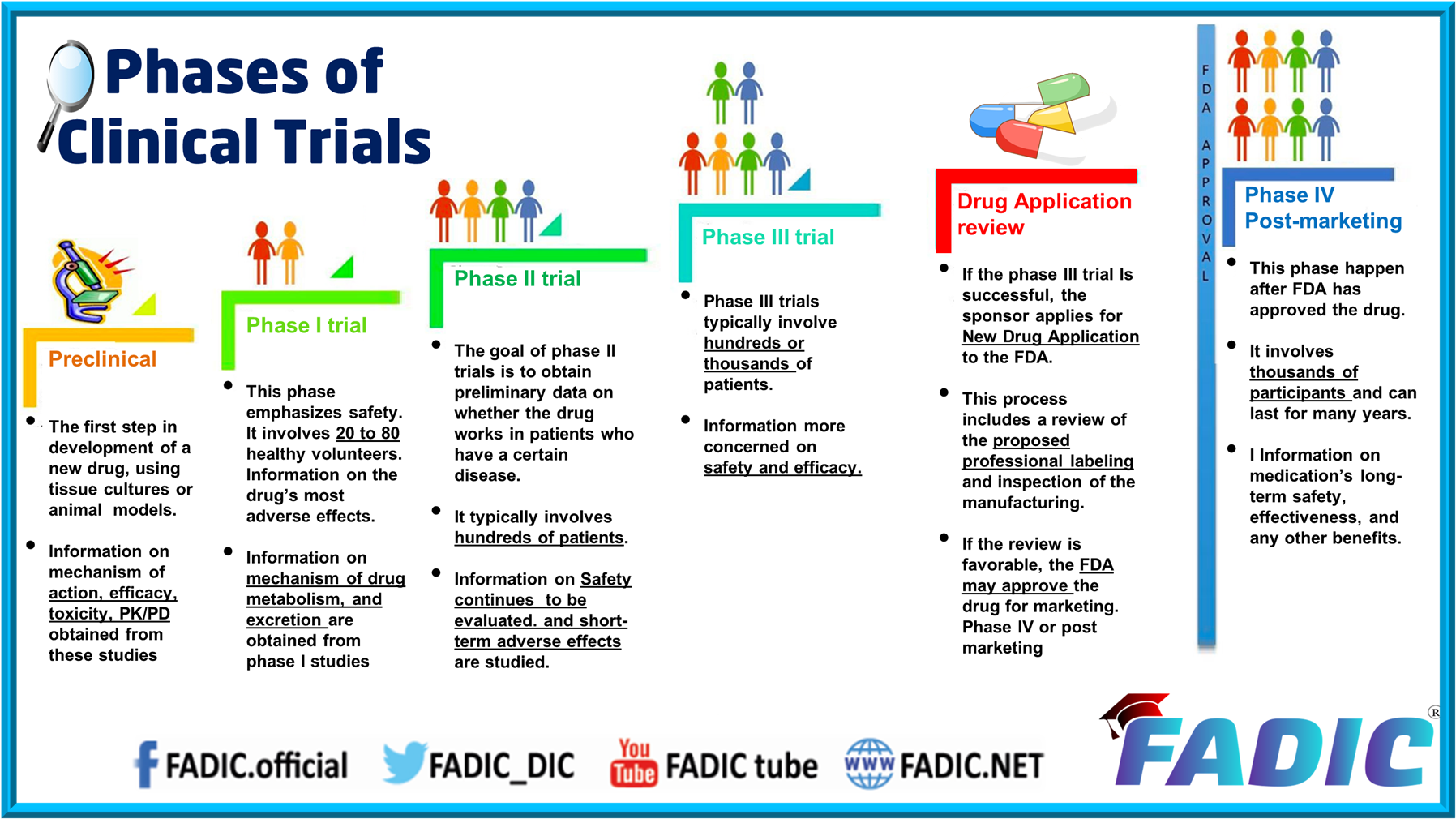
Phases of Clinical Trials 30Minutes Ecourse
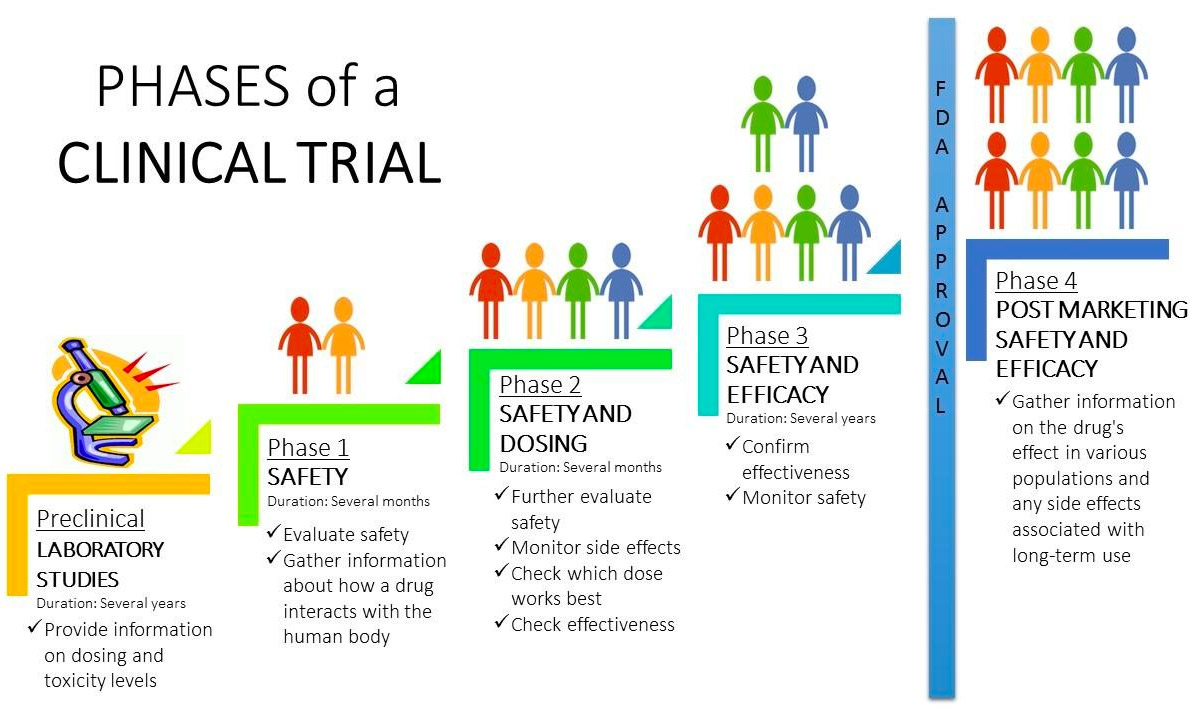
Clinical Trial Phases Diagram
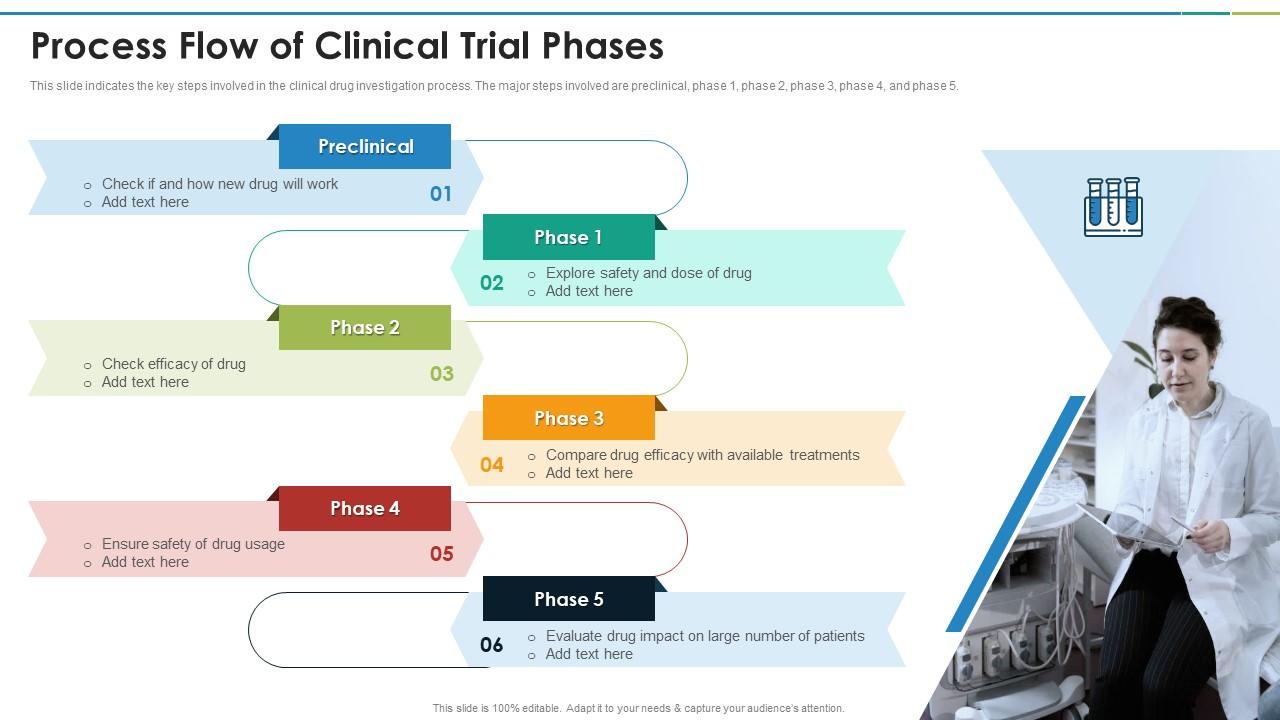
Process Flow Of Clinical Trial Phases Presentation Graphics

Phases Of Clinical Trials Chart
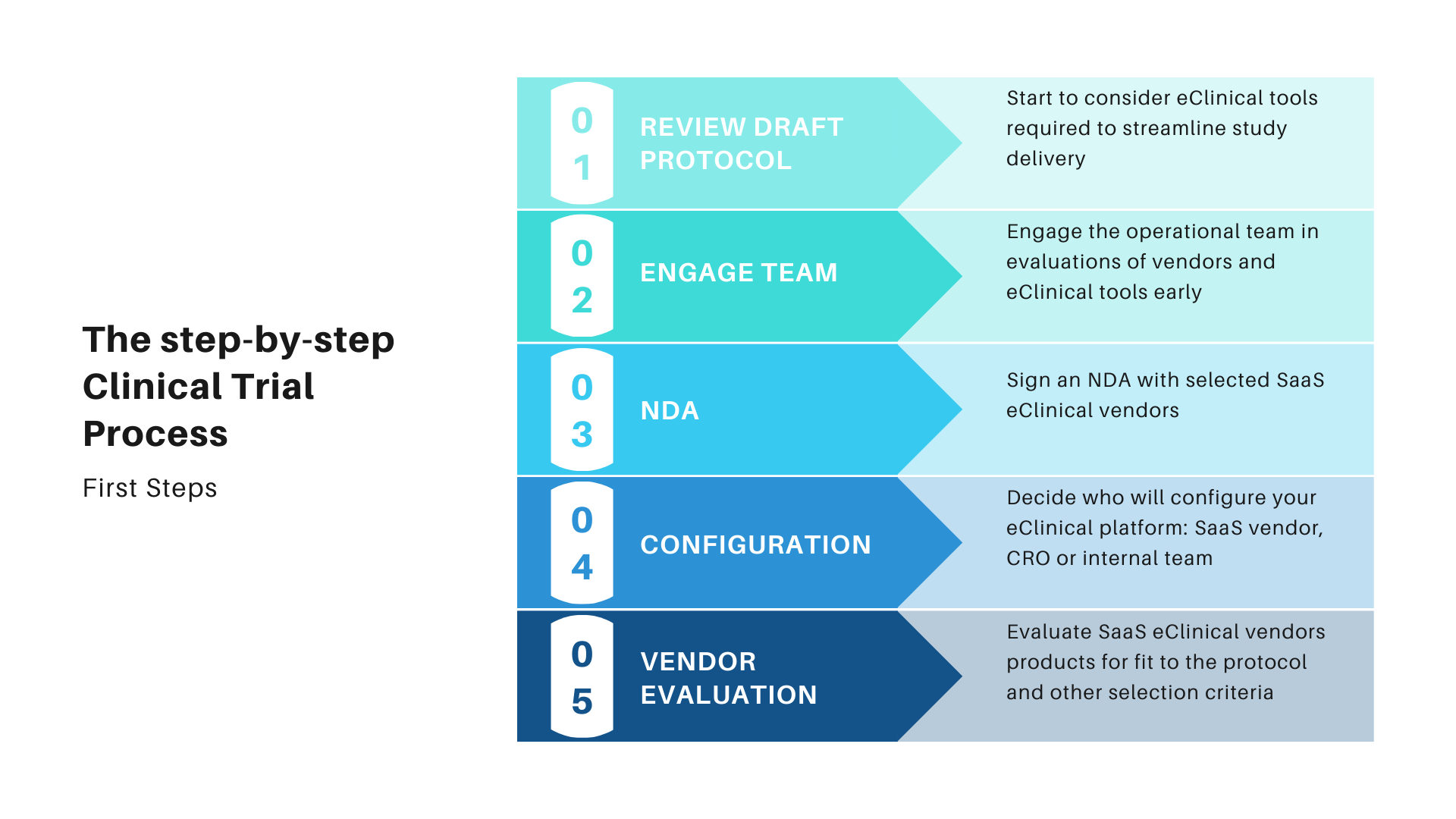
The Clincial Trial Process Stepbystep approach
Doctors Do A Phase I Clinical Trial To Learn If A New Drug, Treatment, Or Treatment Combination Is Safe For People.
They Contribute Significantly To Relevant Research Evidence Developed By The Nihr To Support The Nhs In England And Other Care Providers.
They May Also Explore Different Dosing Schedules Or New Drug Combinations.
Each Phase Is Designed To Answer Certain Questions.
Related Post: