Co2 Temperature Pressure Chart
Co2 Temperature Pressure Chart - 2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. Carbonation is a function of time, temperature, and pressure. Copyright © 2011 by scrivener publishing llc. Thermal conductivity is a material property that describes ability to conduct heat. The 3d structure may be viewed using java or javascript. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °c temperature and 3483 kpa pressure. Distinct liquid and vapor states do not exist. Web gas phase kinetics database. This video shows the property of carbon dioxide to go into a supercritical state with increasing temperature. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Thermal conductivity is a material property that describes ability to conduct heat. What are its triple point and critical point. Web gas phase kinetics database. Web the solid phase is favored at low temperature and high pressure; Copyright © 2011 by scrivener publishing llc. To do this, you will need a regulator for your co2 cylinder that can be adjusted. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °c temperature and 3483 kpa pressure. Copyright © 2011 by scrivener publishing llc. Thermal conductivity is a material property that describes ability to conduct heat. Web ****. Supercritical carbon dioxide ( sco. The 3d structure may be viewed using java or javascript. Web the solid phase is favored at low temperature and high pressure; The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. Thermal conductivity can be defined as. Copyright © 2011 by scrivener publishing llc. Sara anwar and john j. 2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. Web calculation of thermodynamic state variables of carbon dioxide at saturation state, boiling curve. Thermal conductivity can be defined as. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °c temperature and 3483 kpa pressure. This structure is also available as a 2d mol file or as a computed 3d sd file. Web the solid phase is favored at low temperature and high pressure; Learn the carbon dioxide (co2) phase diagram. Distinct. Supercritical carbon dioxide ( sco. Sara anwar and john j. Data at nist subscription sites: Thermal conductivity can be defined as. Web calculation of thermodynamic state variables of carbon dioxide at saturation state, boiling curve. Copyright © 2011 by scrivener publishing llc. Nist / trc web thermo tables, lite edition (thermophysical and thermochemical data) Sara anwar and john j. Thermal conductivity can be defined as. This video shows the property of carbon dioxide to go into a supercritical state with increasing temperature. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. That a system might be subject to. Carbonation is a function of time, temperature, and pressure. Force carbonation is done by infusing (“forcing”) carbon dioxide (co2), a gas, into your beer. The gas phase is favored at high. That a system might be subject to. Web calculate online thermodynamic and transport properties of carbon dioxide based on industrial formulation (formulated in helmholtz energy) for advanced technical applications. Web **** 31.1°c is the critical point of co2, pressure 72.82 bar. To do this, you will need a regulator for your co2 cylinder that can be adjusted. 2) is a. What are its triple point and critical point. Data at nist subscription sites: This structure is also available as a 2d mol file or as a computed 3d sd file. Copyright © 2011 by scrivener publishing llc. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. This structure is also available as a 2d mol file or as a computed 3d sd file. Web the table below gives thermodynamic data of liquid co 2 in equilibrium with its vapor at various temperatures. Copyright © 2011 by scrivener publishing llc. Supercritical carbon dioxide ( sco. 2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. Web calculation of thermodynamic state variables of carbon dioxide at saturation state, boiling curve. Sara anwar and john j. Thermal conductivity can be defined as. Thermal conductivity is a material property that describes ability to conduct heat. The gas phase is favored at high temperature and low pressure. Nist / trc web thermo tables, lite edition (thermophysical and thermochemical data) Copyright © 2011 by scrivener publishing llc. Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °c temperature and 3483 kpa pressure. Distinct liquid and vapor states do not exist. That a system might be subject to.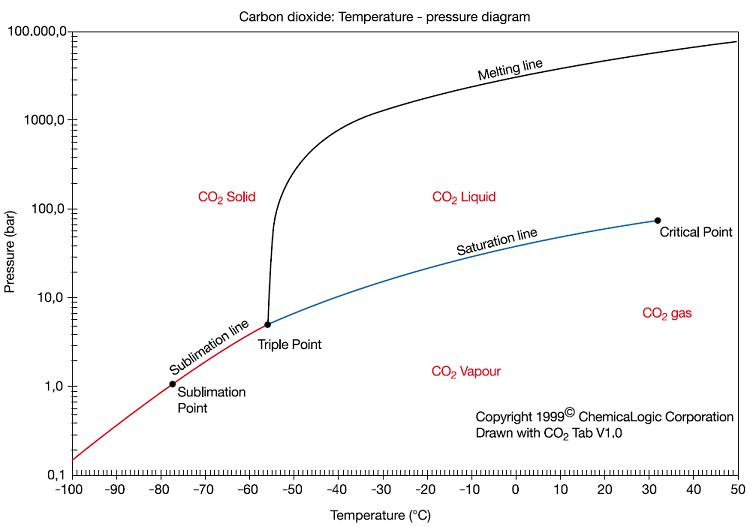
CO2 Pressure Temperature Chart
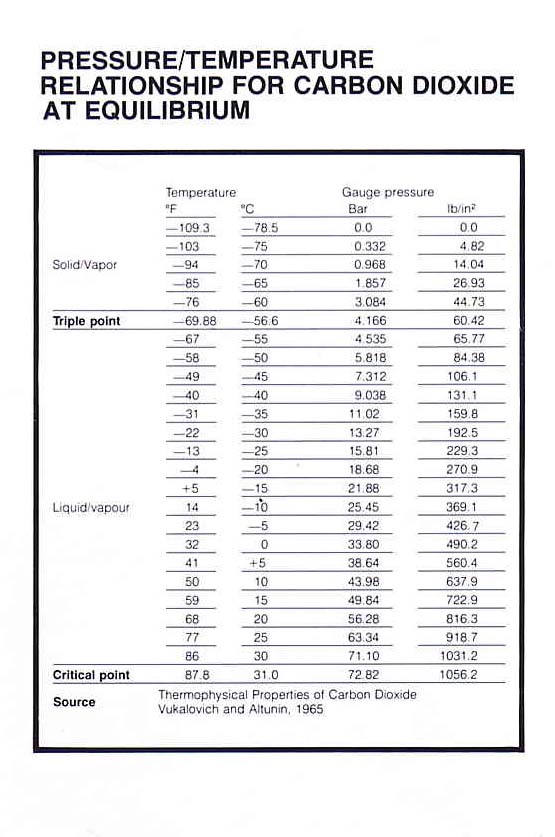
CO 2 Questions
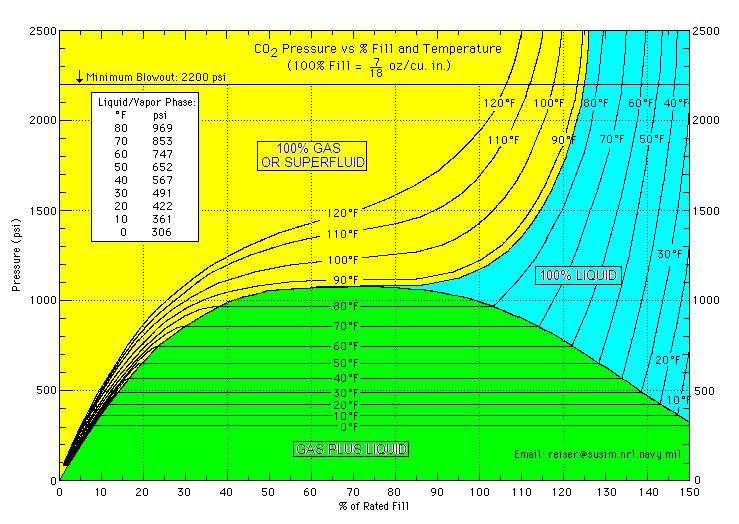
12 gram CO2 cartridge SpudFiles Wiki
![Pressure and temperature phase diagram of CO2 [158,159]. K Kelvin, p](https://www.researchgate.net/publication/314243271/figure/fig4/AS:468921473081347@1488810999637/Pressure-and-temperature-phase-diagram-of-CO2-158-159-K-Kelvin-p-pressure-T.png)
Pressure and temperature phase diagram of CO2 [158,159]. K Kelvin, p

Co2 Pressure And Temperature Chart A Visual Reference of Charts
Co2 Temperature Pressure Chart

CO2 Pressure Temperature Chart

Co2 Pressure Temperature Chart

CO2 Pressure Temperature Chart
Co2 Temp Pressure Chart
Web The Solid Phase Is Favored At Low Temperature And High Pressure;
Web Gas Phase Kinetics Database.
What Are Its Triple Point And Critical Point.
This Video Shows The Property Of Carbon Dioxide To Go Into A Supercritical State With Increasing Temperature.
Related Post: