Draw A Detailed Stepwise Mechanism For The Transesterification
Draw A Detailed Stepwise Mechanism For The Transesterification - Introduction development in the field of renewable energy generation. The sodium methoxide (naome) reacts with the glycerol to form the glycerol alkoxide intermediate. Draw a detailed, stepwise mechanism for the transesterification reaction that forms the biodiesel product in this reaction. Burn fuels more efficiently than gasoline. Let us help you simplify your studying. Web the method being described here is for making fames biodiesel. Web our videos prepare you to succeed in your college classes. Mechanism under basic conditions step 1. You may use a general representation of the triglyceride as shown below in. See the transesterification mechanism and learn how the transesterification reaction occurs under acidic and basic conditions. Web ethyl acetate is a valuable organic solvent and can be prepared by the esterification shown below. You may use a general representation of the triglyceride as shown below in. Less refined than gasoline = less expensive to produce. From there they are mixed methyl esters from which crude. The sodium methoxide (naome) reacts with the glycerol to form the. You may use a general representation of the triglyceride as shown below in. Web chemistry chemistry questions and answers 4. See the transesterification mechanism and learn how the transesterification reaction occurs under acidic and basic conditions. From there they are mixed methyl esters from which crude. The most common method of. The reaction is called transesterification, and the process takes place in four steps. The most common method of. Higher energy density than gasoline. You may use a generic representation of. Questions tips & thanks want to join the conversation? Web the method being described here is for making fames biodiesel. See the transesterification mechanism and learn how the transesterification reaction occurs under acidic and basic conditions. Web chemistry chemistry questions and answers 4. Draw all missing reactants and/or. Draw a detailed stepwise mechanism for the transesterification reaction. Draw all missing reactants and/or. Let us help you simplify your studying. 9) 10) provide the major organic. Oil, alcohol, and a catalyst undergo transesterification. See the transesterification mechanism and learn how the transesterification reaction occurs under acidic and basic conditions. Mechanism under basic conditions step 1. You may use a generic representation of. You may use a general representation of the triglyceride as shown below in. From there they are mixed methyl esters from which crude. Let us help you simplify your studying. The glycerol alkoxide intermediate then. Oil, alcohol, and a catalyst undergo transesterification. Web chemistry chemistry questions and answers 4. Transesterification is the conversion of a carboxylic acid ester into a different carboxylic acid ester. Draw a detailed, stepwise mechanism for the transesterification reaction that forms the biodiesel product in this reaction. Web learn what transesterification is. Higher energy density than gasoline. You may use a generic representation of. Less refined than gasoline = less expensive to produce. In the transesterification mechanism, the carbonyl carbon of the starting ester reacts to give a tetrahedral intermediate, which either reverts back to the starting. Web ethyl acetate is a valuable organic solvent and can be prepared by the esterification shown below. Web schematic of the biodiesel process using transesterification: Web draw a detailed stepwise mechanism for the transesterification reaction that forms the biodiesel. Has a higher boiling point. Write the complete stepwise mechanism for this reaction. The alcohol is deprotonated by the basic medium, resulting in the formation of an alkoxide ion. You may use a generic representation of. Transesterification is the conversion of a carboxylic acid ester into a different carboxylic acid ester. Let us help you simplify your studying. 9) 10) provide the major organic. Web the stepwise mechanism for the transesterification reaction that forms biodiesel from a triglyceride is as follows: Less refined than gasoline = less expensive to produce. In the transesterification mechanism, the carbonyl carbon of the starting ester reacts to give a tetrahedral intermediate, which either reverts back to the starting. The reaction is called transesterification, and the process takes place in four steps. Write the complete stepwise mechanism for this reaction. The glycerol alkoxide intermediate then. Introduction development in the field of renewable energy generation. Questions tips & thanks want to join the conversation? Has a higher boiling point. Let us help you simplify your studying. You may use a generic representation of. Draw a detailed, stepwise mechanism for the transesterification reaction that forms the biodiesel product in this reaction. Mechanism under basic conditions step 1. Draw a detailed stepwise mechanism for the transesterification reaction. The most common method of. Web learn what transesterification is.Proposed reaction mechanism 1 for transesterification reactions in the

8 Mechanism of basecatalyzed transesterification. Download

General equation of transesterification. b Basic reaction mechanism for
[Solved] . 4. Draw a detailed stepwise mechanism for the given reaction
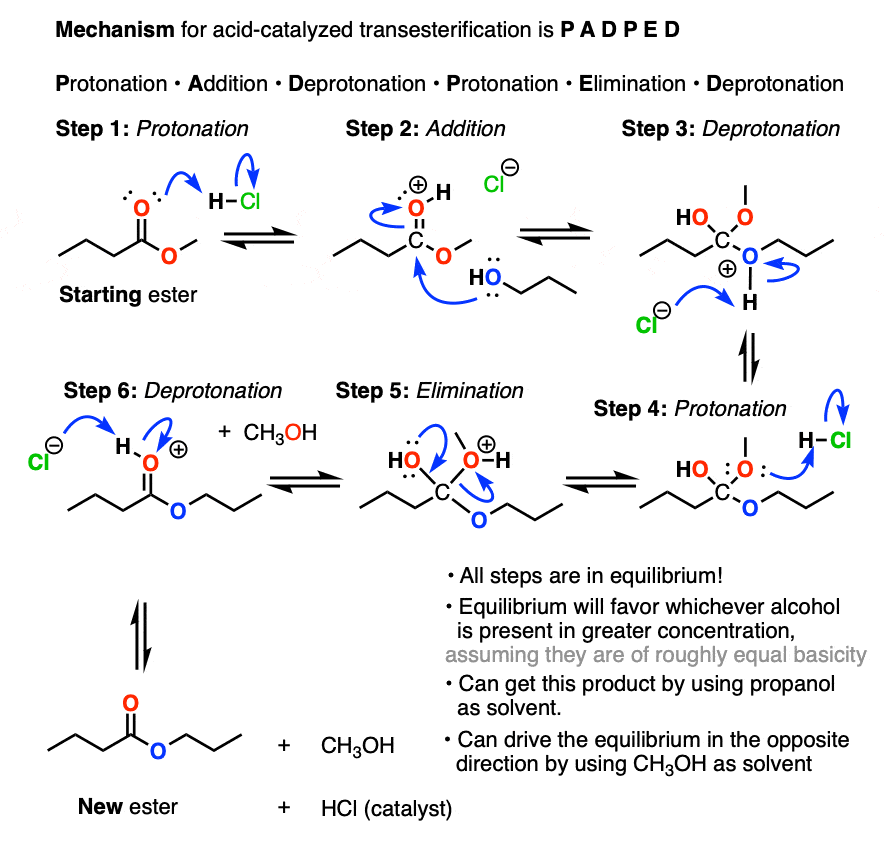
Transesterification Master Organic Chemistry

Transesterification Chemistry Steps
Reaction mechanism for base catalyzed transesterification Download
[Solved] Write a detailed reaction mechanism for the Fischer

Mechanism of base catalyzed transesterification. Download Scientific

Plausible mechanism for the transesterification reaction of 2 PA and
Web Schematic Of The Biodiesel Process Using Transesterification:
Web Our Videos Prepare You To Succeed In Your College Classes.
The Alcohol Is Deprotonated By The Basic Medium, Resulting In The Formation Of An Alkoxide Ion.
From There They Are Mixed Methyl Esters From Which Crude.
Related Post: