Draw A Lewis Structure For Sicl4
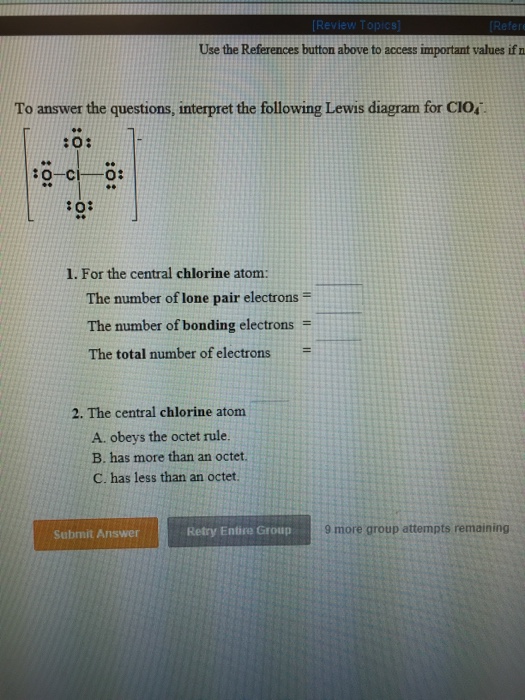
Draw A Lewis Structure For Sicl4 - Steps for writing lewis structures. (a) nitrate ion ([n o3]−) (b) phosphoric acid (h 3p o4) (c) aluminum chloride (alcl3) (d) sodium phosphate (n a3p o4) view solution click here:point_up_2:to get an answer to your question :writing_hand:draw the lewis structures for the following molecules and ions Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Web how to draw lewis structure for sicl 4? Determine the total number of valence electrons in the molecule or ion. Silicon (si) and chlorine have four and seven electrons in. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Include all lone pairs of electrons. What is the total number of valence electrons in the lewis structure of sicia? The central silicon atom _ a. The electron pair geometry at the central atom c. • if the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. For the sicl4 structure use the periodic table to find the total number of valence. Web let's do the sicl4 lewis structure. (a). Discover the electron dot structure and valence electrons for sicl4, which will help you understand its chemical properties and reactions. While selecting the center atom, always put the least electronegative atom at the. Count total valence electron in sicl4 of silicon and chlorine atoms through the periodic group number. #4 calculate formal charge and check stability (if octet is already. Web the first step is to sketch the lewis structure of the sicl4 molecule, to add valence electron around the silicon atom; • if the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. Draw a lewis structure for sicl4 in which the central. We've got four cl's there, so if we multiply that all together we've got 28 plus 4: While selecting the center atom, always put the least electronegative atom at the. Web let's do the sicl4 lewis structure. Count total valence electron in sicl4 of silicon and chlorine atoms through the periodic group number. The electron pair geometry at the central. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web by using the following steps, you can easily draw the lewis structure of sicl 4: Web chemistry questions and answers. The number of lone pairs = the number of this problem has been solved! For the central silicon atom: The central si atom forms single bonds the central si atom forms double bonds to answer the. Web use these steps to correctly draw the sicl 4 lewis structure: • if the species contains oxygen, do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. Draw the molecule. #1 first draw a rough sketch first, determine the total number of valence electrons periodic table Web let's do the sicl4 lewis structure. Web how to draw lewis structure for sicl 4? Si is in group 4 or 14 on the periodic table, so it has 4 valence electrons. The hybridization of the central atom; Find the total valence electrons in sicl4 molecule in order to find the total valence electrons in sicl4 molecule, first of all you should know the valence electrons. Web steps of drawing lewis structure of sicl 4 total number of electrons of the valance shells of sicl 4. The central si atom forms single bonds the central si atom forms. Web chemistry questions and answers. Total electron pairs are determined by dividing the number total valence electrons by two. Draw the molecule by placing atoms on the grid and connecting them with bonds. Draw a lewis structure for sicl4 in which the central si atom obeys the octet rule, and answer the following questions bases on your drawing. Web how. Draw a lewis structure for sicl4 in which the central si atom obeys the octet rule, and answer the following questions bases on your drawing. Include all lone pairs of electrons. Discover the electron dot structure and valence electrons for sicl4, which will help you understand its chemical properties and reactions. Calculate the total number of valence electrons. The central. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. What is the total number of valence electrons in the lewis structure of sicia? We've got four cl's there, so if we multiply that all together we've got 28 plus 4: The central silicon atom _ a. Si is in group 4 or 14 on the periodic table, so it has 4 valence electrons. Web how to draw lewis structure for sicl 4? Silicon (si) and chlorine have four and seven electrons in. Draw the molecule by placing atoms on the grid and connecting them with bonds. Electrons draw a lewis structure for sic14. С р opy aste [+ с chemdoodle previous. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Draw a lewis structure for sicl4 in which the central si atom obeys the octet rule, and answer the following questions bases on your drawing. Web there is no lone pair present on the central atom in the sicl4 lewis structure. Determine the total number of valence electrons in the molecule or ion. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Count total valence electron in sicl4 of silicon and chlorine atoms through the periodic group number.
How to draw a SiCl4 Lewis Structure? Science Education and Tutorials
[Solved] 4. a. (3 points) Draw the Lewis structure for SiCl4. b. (3
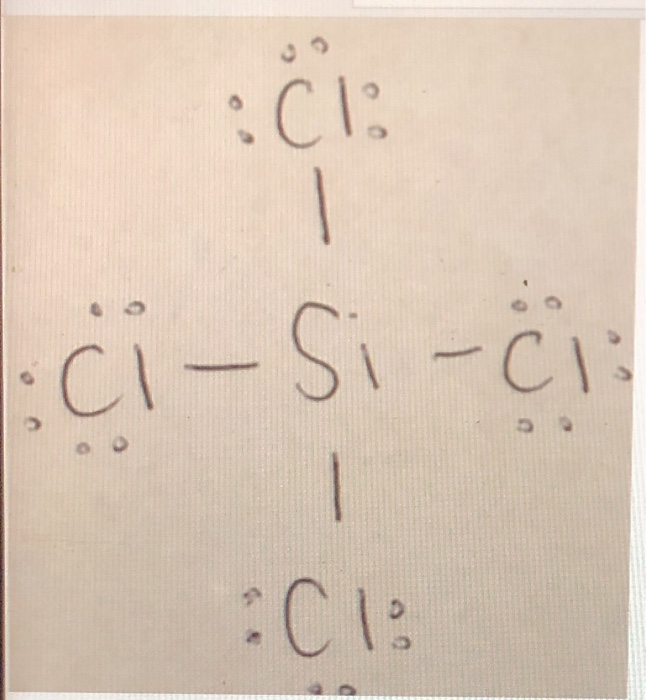
Solved uestion 4 In the Lewis structure for SiCl4. what is

SiCl4 Lewis Structure (Silicon Tetrachloride) YouTube

Molecular Structure Sicl4

Draw the Lewis structures for the following molecules and ions H2S
[Solved] 4. a. (3 points) Draw the Lewis structure for SiCl4. b. (3

How to draw a SiCl4 Lewis Structure? Science Education and Tutorials

SiCl4 Molecular Geometry Science Education and Tutorials
Solved Draw a Lewis structure for SiCl4 in which the central
(A) Nitrate Ion ([N O3]−) (B) Phosphoric Acid (H 3P O4) (C) Aluminum Chloride (Alcl3) (D) Sodium Phosphate (N A3P O4) View Solution Click Here:point_Up_2:To Get An Answer To Your Question :Writing_Hand:draw The Lewis Structures For The Following Molecules And Ions
The Next One Is To.
Include All Lone Pairs Of Electrons.
Web Draw The Lewis Structure For Sih4 And Give The Following:
Related Post: