Draw A Resonance Structure For The Following Cation.
Draw A Resonance Structure For The Following Cation. - Web draw resonance structures of the cation shown below: First, let's draw the given cation: Web this problem has been solved! Web draw the resonance structures of molecules or ions that exhibit delocalization. Be sure to include the formal charge on structures and c. Web draw the resonance structures of molecules or ions that exhibit delocalization. The total formal charge is +6. Draw a resonance structure for the. Resonating structures are the structures that are different in position. There are six electron pairs, three in the 1s orbital and three in the 2s orbital. Web this problem has been solved! Web the equivalent ressonance structures seem like the same but there are non equivalent ressonance strutures that occur when the delocalization of electrons is between qualitativity. Web draw resonance structures of the cation shown below: First, let's draw the given cation: You'll get a detailed solution from a subject matter expert that helps you. Be sure to include the formal. The total formal charge is +6. Draw a resonance structure for the. In order to draw the resonance structures, one has to keep the following rules in mind: Shifting only one electron pair in each step. Web this problem has been solved! Web draw the resonance structures of molecules or ions that exhibit delocalization. Web 1) for the following resonance structures please rank them in order of stability. Indicate which structure makes the largest contribution to the. Be sure to include the formal charge on structures and c. Web chemistry chemistry questions and answers (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. Web this problem has been solved! Shifting only one electron pair in each step. First, let's draw the given cation: Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Determine the relative stability of resonance structures using a set of. In order to draw the resonance structures, one has to keep the following rules in mind: First, let's draw the given cation: Now, let's draw all possible resonance. Web this problem has been solved! There are six electron pairs, three in the 1s orbital and three in the 2s orbital. Web draw resonance structures of the cation shown below: The total formal charge is +6. Just started with resonance, where would… a: Determine the relative stability of resonance structures using a set of rules. Now, let's draw all possible resonance. Draw a resonance structure for the. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Web this problem has been solved! Indicate which would be the major contributor to the resonance hybrid. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Indicate which would be the major contributor to the resonance hybrid. Web draw resonance structures of the cation shown below: There are six electron pairs, three in the 1s orbital and three in the 2s. Draw the resonance structure for the following molecule: Now, let's draw all possible resonance. Determine the relative stability of resonance structures using a set of. Web this problem has been solved! Resonating structures are the structures that are different in position. Web 1) for the following resonance structures please rank them in order of stability. Determine the relative stability of resonance structures using a set of. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the resonance structures of molecules or ions that exhibit delocalization. There are six electron pairs, three in. There are six electron pairs, three in the 1s orbital and three in the 2s orbital. Web chemistry chemistry questions and answers (a) draw two resonance structures of the cation shown, shifting only one electron pair in each step. Determine the relative stability of resonance structures using a set of rules. Web 1) for the following resonance structures please rank them in order of stability. Web this problem has been solved! Indicate which structure makes the largest contribution to the. Shifting only one electron pair in each step. Be sure to include the formal charge on structures and c. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Resonance is a mental exercise and method. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Indicate which would be the major contributor to the resonance hybrid. Web draw the resonance structures of molecules or ions that exhibit delocalization. Draw a resonance structure for the. Be sure to include the formal. Determine the relative stability of resonance structures using a set of.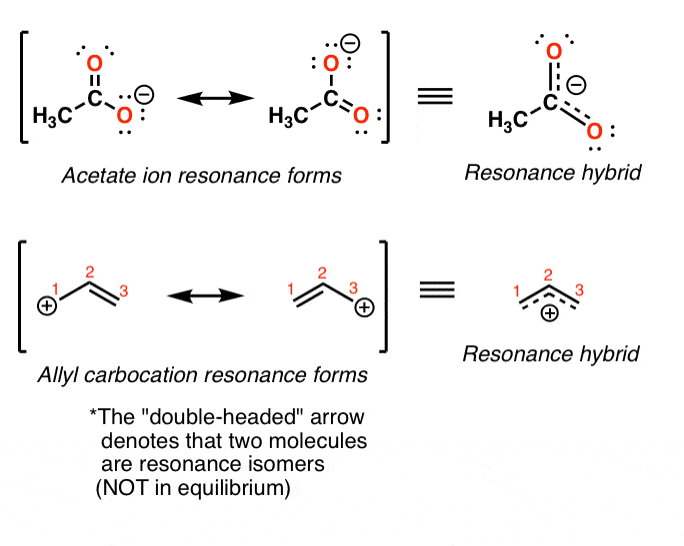
Resonance Structures 4 Rules On How To Evaluate Them, With Practice
draw two resonance structures of the cation shown below
draw two resonance structures of the cation shown below
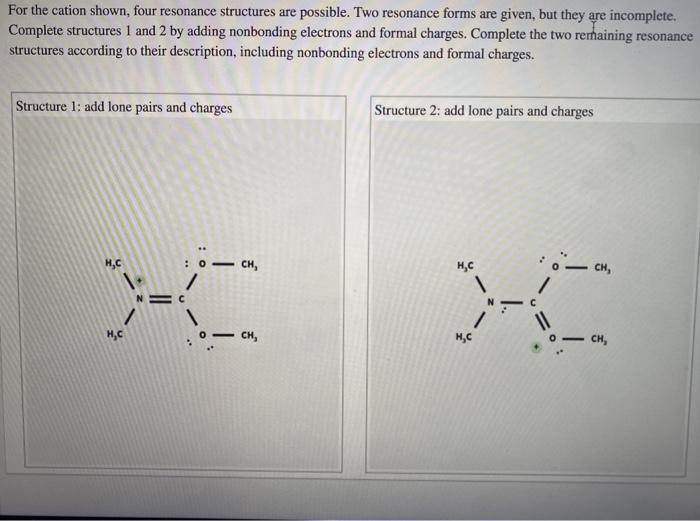
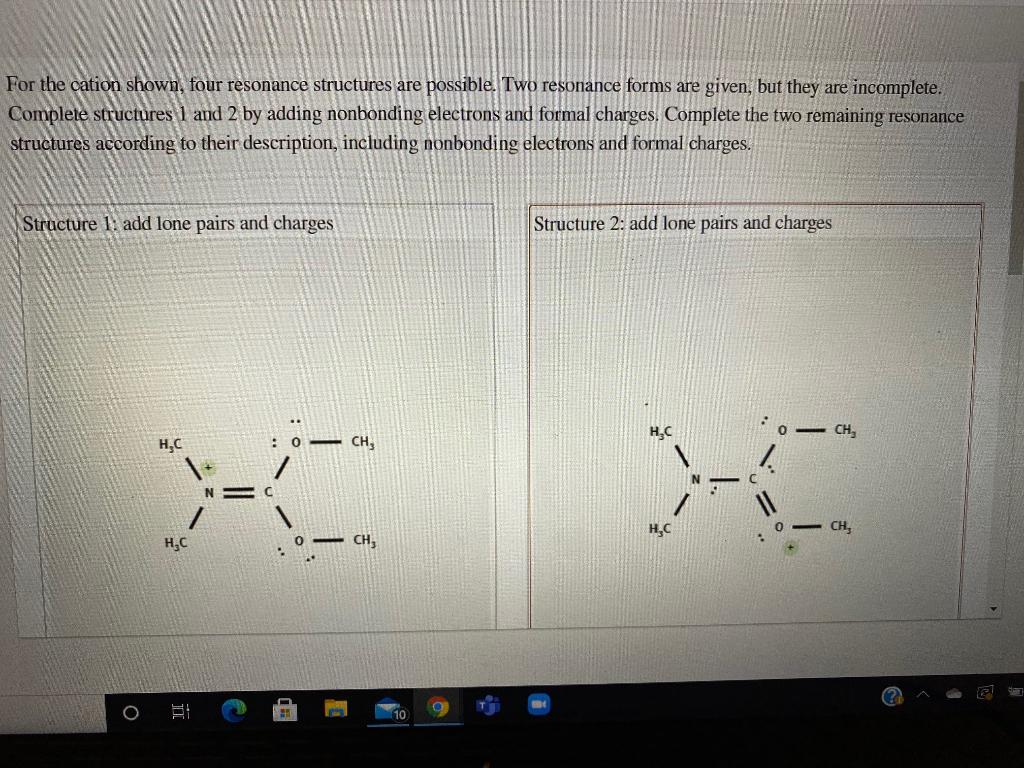
Solved For the cation shown, four resonance structures are
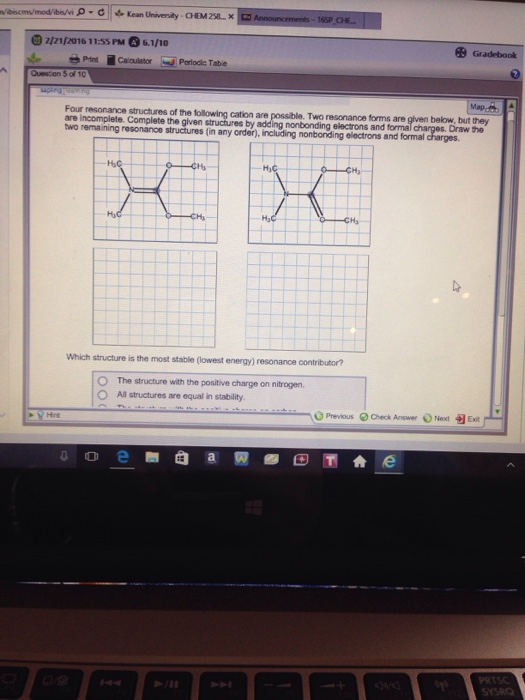
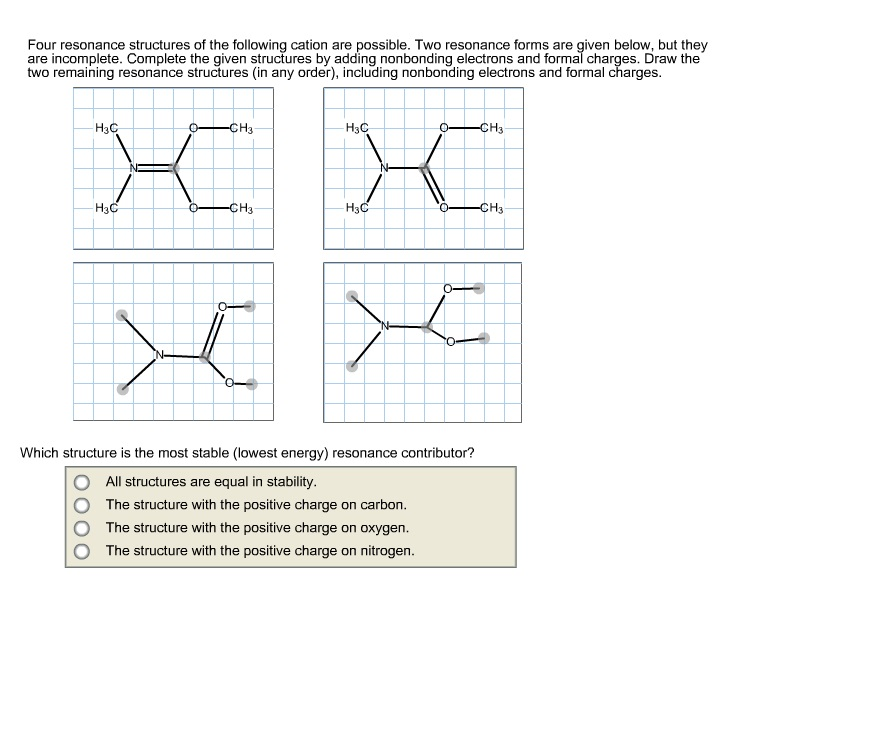
Solved Four resonance structure of the following cation are
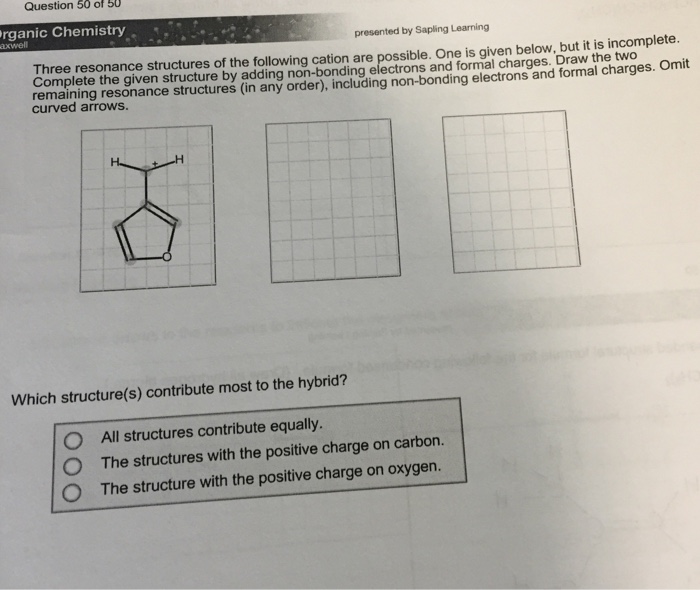
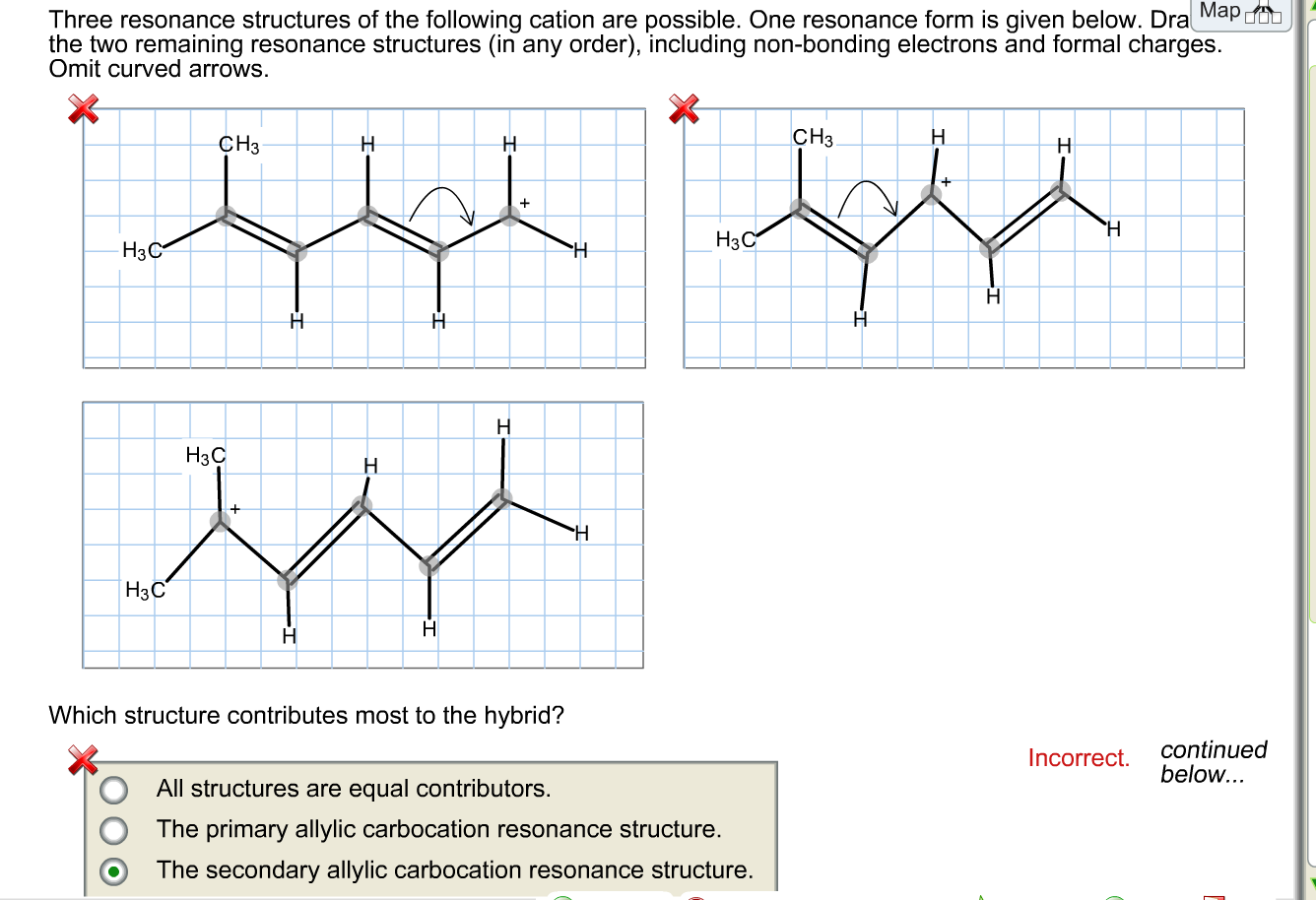
Solved Three resonance structures of the following cation

OneClass C) Draw the resonance structure 3) Draw the resonance
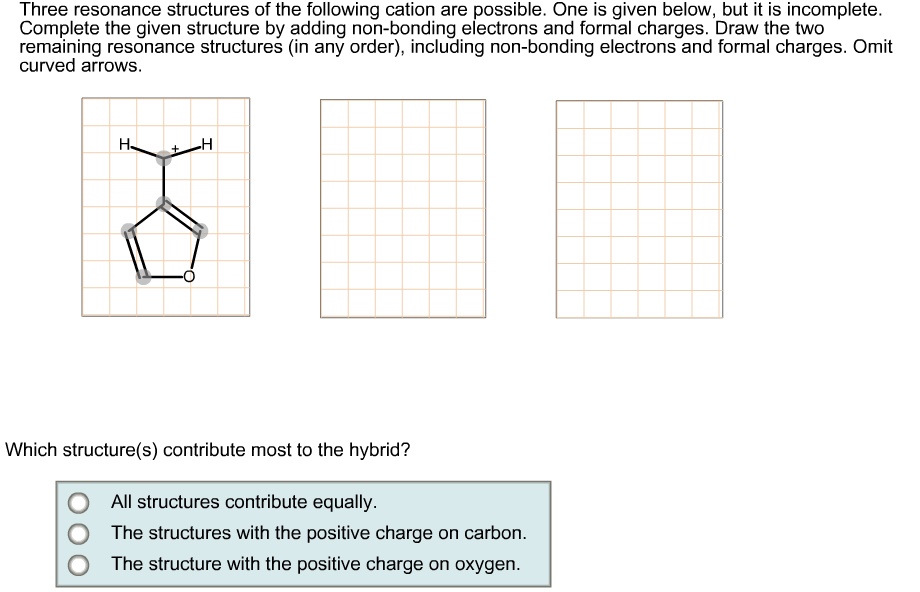
SOLVED Three resonance structures of the following cation are possible
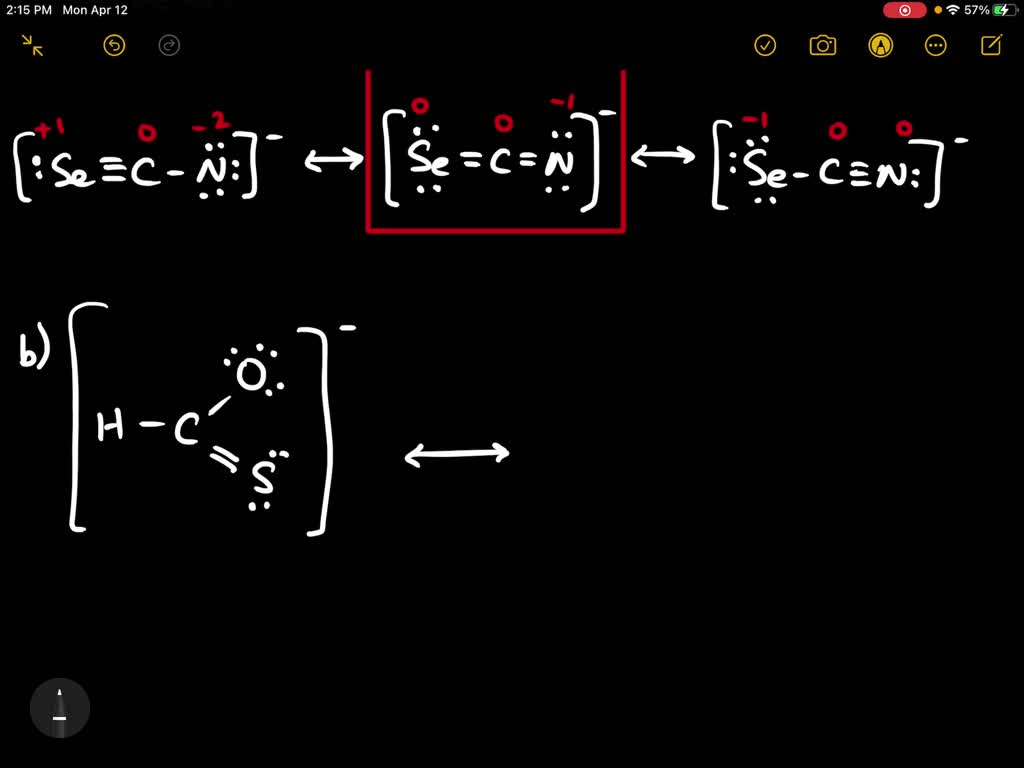
Four resonance structures of the following cation are… SolvedLib
Solved For the cation shown, four resonance structures are
Web The Equivalent Ressonance Structures Seem Like The Same But There Are Non Equivalent Ressonance Strutures That Occur When The Delocalization Of Electrons Is Between Qualitativity.
In Order To Draw The Resonance Structures, One Has To Keep The Following Rules In Mind:
Web Draw The Resonance Structures Of Molecules Or Ions That Exhibit Delocalization.
Web (A) Draw The Resonance Structures For The Following Cation:
Related Post: