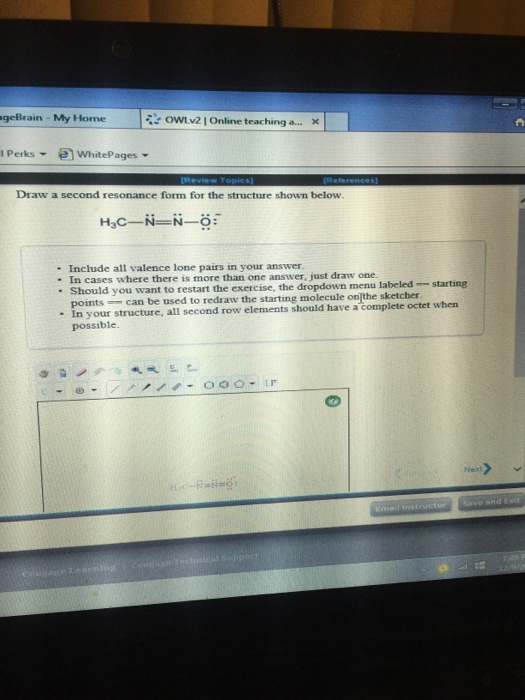
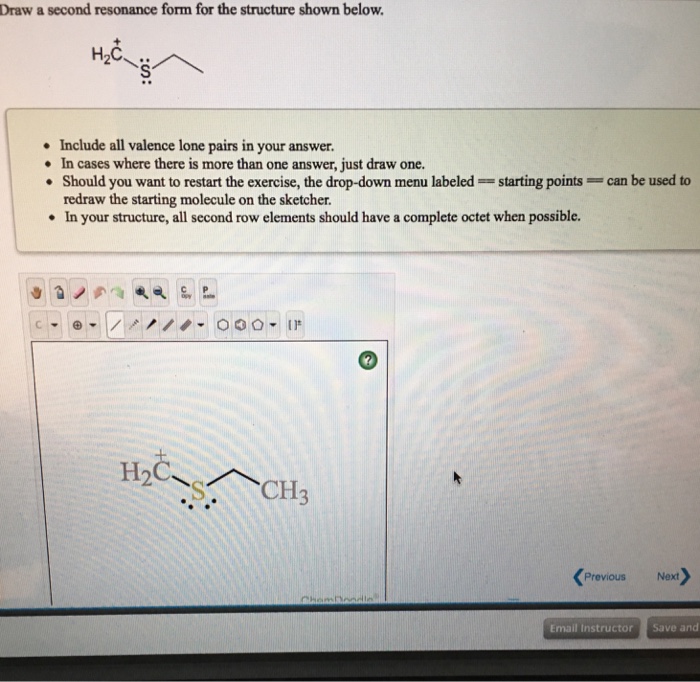
Draw A Second Resonance Form For The Structure Shown Below
Draw A Second Resonance Form For The Structure Shown Below - Equivalent lewis structures are called resonance forms. Use the concept of resonance to explain structural features of molecules and ions. They are used when there is more than one way to place double bonds and lone pairs on atoms. Buy chemical principles in the laboratory 11th edition isbn: Hello, so the second resonant form of this structure is going to look like this we're going to have. So remember that resonance structures are just going to move and delocalize electrons. Draw a second resonance form for the structure shown below. Web h2c draw a second resonance form for the structure shown below ch hoc this problem has been solved! 2) draw four additional resonance contributors for the molecule below. Cengage learning expand_more chapter 1 : This pattern is always done for residents. Web resonance form for the structure shown below. Buy chemistry 10th edition isbn: • in cases where there is more than one answer, just draw one. We're going to have a single we're going to have Web science chemistry chemistry questions and answers 1. Web the resulting structure is the second resonance form. Draw a second resonance form for the structure shown below. Use the concept of resonance to explain structural features of molecules and ions. Buy chemistry 10th edition isbn: Web draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown below. Web 1) for the following resonance structures please rank them in order of stability. Draw a second resonance form for each of the structure. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. With resonance, we can shift electrons in their positions, but we still need to be careful. Draw a second resonance form for each of the structure. Draw a second resonance form for the structure shown below. Cengage learning expand_more chapter 5 : Draw a second resonance structure for the below species. This pattern is always done for residents. Equivalent lewis structures are called resonance forms. Indicate which would be the major contributor to the resonance hybrid. Draw both resonance forms for the enolate ions of the carbonyl compounds. They are used when there is more than one way to place double bonds and lone pairs on atoms. Web 1) for the following resonance structures please rank them in order of stability. Web science chemistry chemistry questions and answers 1. With resonance, we can shift electrons in their positions, but we still need to be careful. A guided inquiry 2nd edition isbn: In this case, we have a nitrogen atom with a lone pair and a carbon atom. Web draw a second resonance form for the structure shown below. Web draw a second resonance structure for the given species below. Cengage learning expand_more chapter 1 : Web first, we need to identify the atoms that can move their electrons to form a double bond or a lone pair. Web draw a second resonance structure and the hybrid for. Determine the relative stability of resonance structures using a set of rules. That is the double bond single single you're, going to have another double bond here: A guided inquiry 2nd edition isbn: 2) draw four additional resonance contributors for the molecule below. Draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown below. Determine the relative stability of resonance structures using a set of rules. Draw a second resonance form for each of the structure. Cengage learning expand_more chapter 1 : Draw a second resonance form for the structure shown below. Buy chemical principles in the laboratory 11th edition isbn: Web science chemistry draw a second resonance form for the structure shown below. Hello, so the second resonant form of this structure is going to look like this we're going to have. Draw a second resonance form for the structure shown below. • in cases where there is more than one. Indicate which would be the major contributor to the resonance hybrid. Web resonance form for the structure shown below. Equivalent lewis structures are called resonance forms. Hello, so the second resonant form of this structure is going to look like this we're going to have. Draw the resonance structure of the following compound. H2c ch2 draw a second resonance form for the structure shown below. Draw a second resonance structure for the below species. H3c nh2 • include all valence lone pairs in your answer. Web the resulting structure is the second resonance form. Web science chemistry chemistry questions and answers 1. Buy chemistry 10th edition isbn: Draw a second resonance form for. However, you can easily draw it based on the steps provided. In this case, we have a nitrogen atom with a lone pair and a carbon atom with a double bond. Web draw a second resonance structure and the hybrid for the following species below, and then rank the two resonance structures and the hybrid in order of increasing stability. Web 1) for the following resonance structures please rank them in order of stability.Solved Draw a second resonance form for the structure shown
Solved Draw a second resonance form for the structure shown
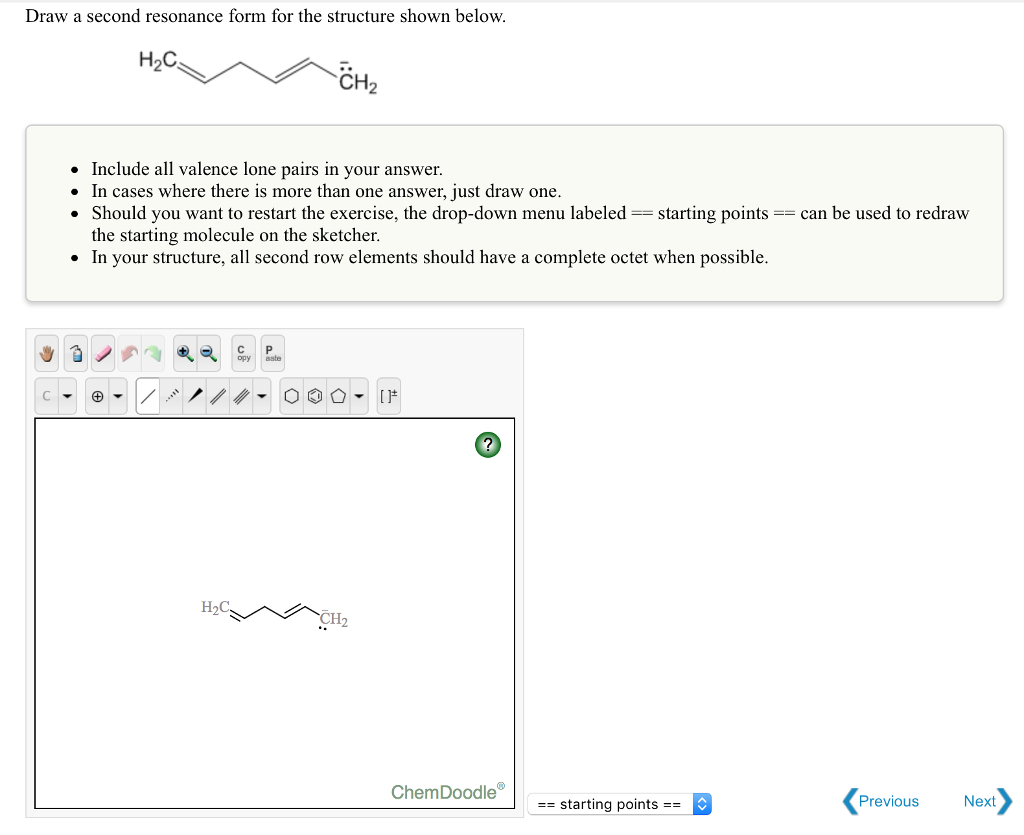
Solved Draw a second resonance form for the structure shown

Solved Draw a second resonance form for the structure shown
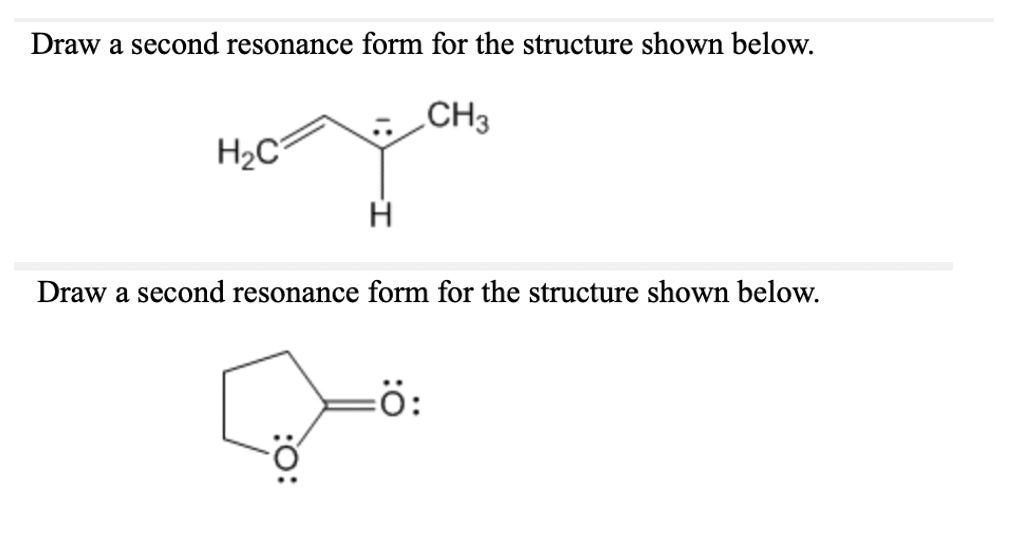
SOLVED Draw a second resonance form for the structure shown below CH3
[Solved] Draw a second resonance form for the structure shown below. H

Solved Draw a second resonance form for the structure shown

OneClass Draw a second resonance form for the structure shown below
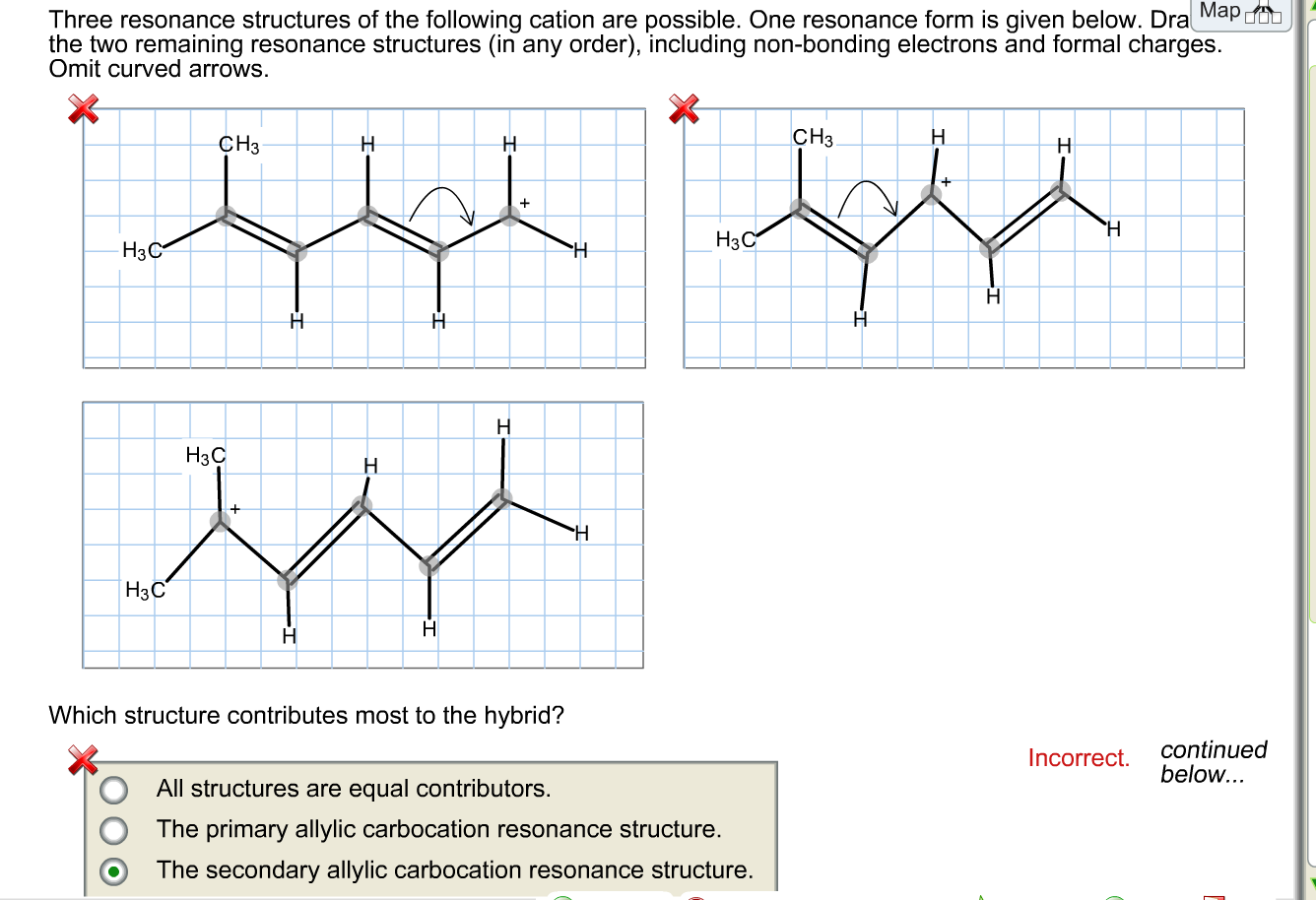
draw two resonance structures of the cation shown below

OneClass Draw a second resonance form for the structure shown below
Web Draw A Second Resonance Form For The Structure Shown Below.
Use The Concept Of Resonance To Explain Structural Features Of Molecules And Ions.
Cengage Learning Expand_More Chapter 5 :
Draw One Resonance Structure For The Compound.
Related Post: