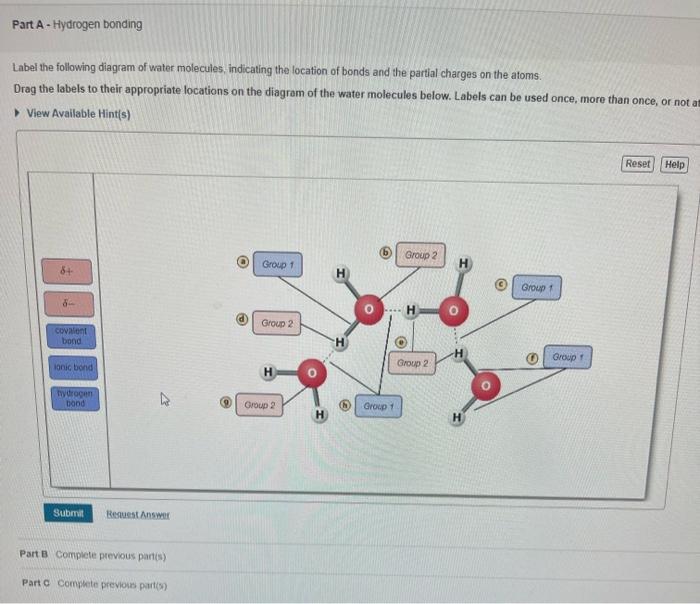
Draw A Water Molecule And Label The Partial Charges
Draw A Water Molecule And Label The Partial Charges - The adjacent water molecules align themselves so that the partial charges on adjacen molecules can form an attraction. Web water's large dipole moment leads to hydrogen bonding. Arrange them and label where hydrogen bonding is occurring. Web the partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. (use flat bohr atoms showing the sharing of electrons in molecules.) electronegativity values are o=3.44, c=2.5, and h=2.1. Since it has the same number of protons and electrons, the water molecule is neutral. In pure liquid water, each h 2 o molecule is hydrogen bonded to an average of 3.4 adjacent h 2 o molecules, compared with 4 in ice. This is illustrated by the gradation in color in the schematic diagram here. Web draw a water molecule with charges. Label each atom and each location of a partial charge. Web partial positive charge and the oxygen atom acquires a partial negative charge. Since it has the same number of protons and electrons, the water molecule is neutral. The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. (use flat bohr atoms showing the sharing of electrons in molecules.) electronegativity values are. Complete the labels showing the locations of the hydrogen atoms, the oxygen atom, and the regions of. (use flat bohr atoms showing the sharing of electrons in molecules.) electronegativity values are o=3.44, c=2.5, and h=2.1. Thus, in an aqueous environment, water molecules, which are at a very high. Because the water molecule has an h — o — h bond. Since it has the same number of protons and electrons, the water molecule is neutral. The partial negative charge of one water molecule is attracted to the partial positive charge of another water molecule. Use a dotted line to represent the hydrogen bond. Web let u= {w, o, m, e, n}, m= {n, o, w}, and t= {w, o, n}.. The partial negative charge of one water molecule is attracted to the partial positive charge of another water molecule. (use flat bohr atoms showing the sharing of electrons in molecules.) electronegativity values are o=3.44, c=2.5, and h=2.1. Draw a ring around an atom or a group of atoms making up an r group that could hydrogen bond with a neighboring. The adjacent water molecules align themselves so that the partial charges on adjacen molecules can form an attraction. Draw a ring around an atom or a group of atoms making up an r group that could hydrogen bond with a neighboring r group. Thus, in an aqueous environment, water molecules, which are at a very high. Sign up and see. One water molecule label all atoms label all polar covalent bonds label all partial charges draw: The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. Start studying label water molecule. Web let u= {w, o, m, e, n}, m= {n, o, w}, and t= {w, o, n}. The adjacent water molecules. Label each atom and each location of a partial charge. The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. Web label charges and draw stick model that represents molecule; Web element that has a slight negative charge when sharing electrons within a water molecule. The partial negative charge at one end. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Label each atom and each location of a partial charge. Use a dotted line to represent the hydrogen bond. The electron cloud model shows where electrons are in a molecule.. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. The electron cloud model shows where electrons are in a molecule. Web partial positive charge and the oxygen atom acquires a partial negative charge. Because the water molecule has an h — o — h bond. Web polar molecules have full or partial charges. This is illustrated by the gradation in color in the schematic diagram here. The adjacent water molecules align themselves so that the partial charges on adjacen molecules can form an attraction. This creates a partial charge called a dipole. Web label charges and draw stick model that represents molecule; Web the water molecule has a total of 10 protons and 10 electrons (8 from the oxygen atom and 1 from each of the two hydrogen atoms). Label the atoms that make up the molecule, as well as their partial charges. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Web partial positive charge and the oxygen atom acquires a partial negative charge. The electronic (negative) charge is concentrated at the oxygen end of the molecule, owing. The bent shape of the water molecule is critical because the polar o−h o − h bonds do not cancel one another and the molecule as a whole is polar. Learn vocabulary, terms, and more with flashcards, games, and other study tools. 1) indicate any full or partial charges, 2) label as symmetrical or asymmetrical, and 3) label as nonpolar or polar. The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. Water is polar due to the uneven sharing of electrons between oxygen and hydrogen. Thus, in an aqueous environment, water molecules, which are at a very high. Start studying label water molecule. The h 2 o molecule is electrically neutral, but the positive and negative charges are not distributed uniformly. Since it has the same number of protons and electrons, the water molecule is neutral. The adjacent water molecules align themselves so that the partial charges on adjacen molecules can form an attraction. Arrange them and label where hydrogen bonding is occurring.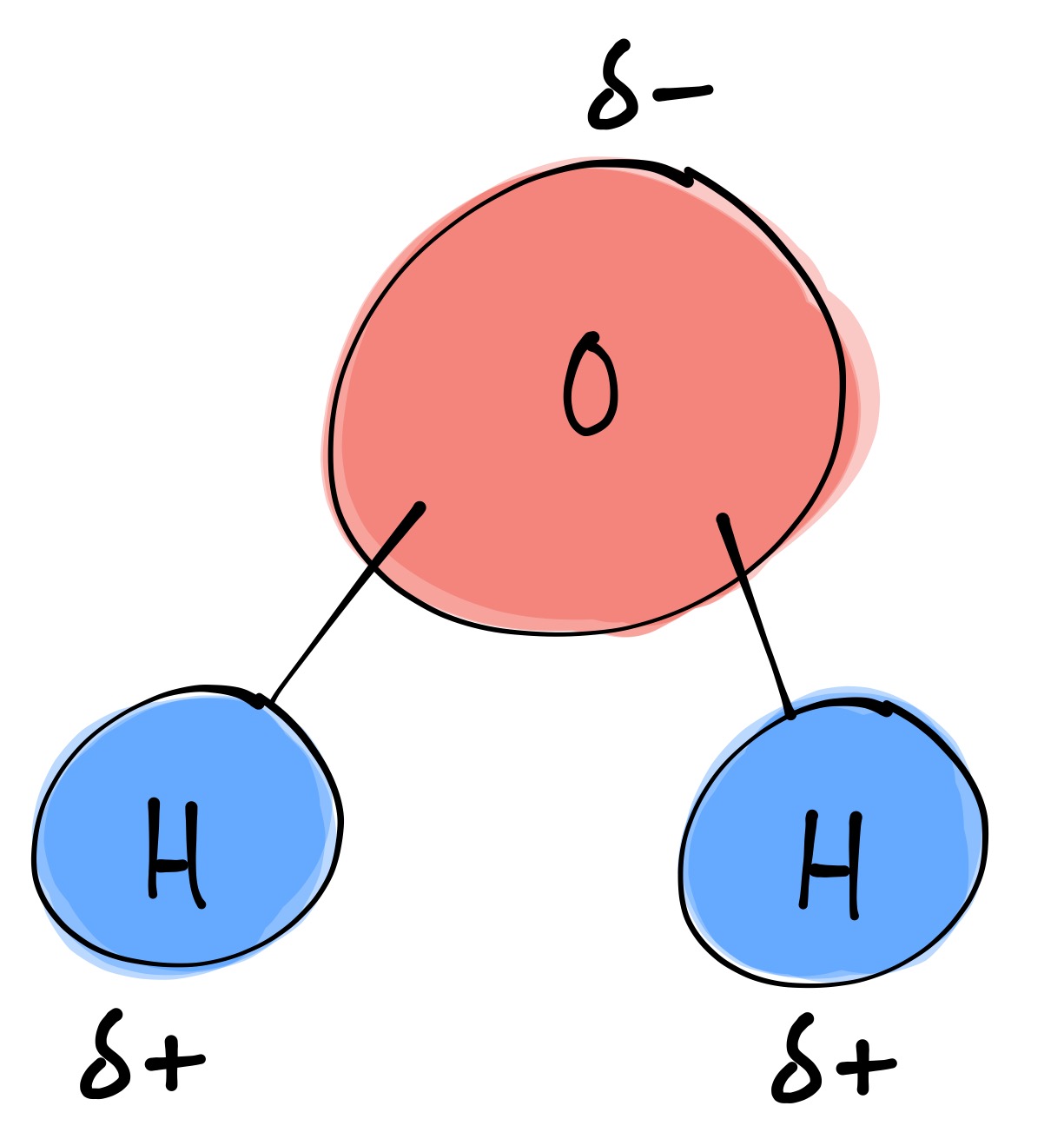
Water Molecule Gay Eat Ass

Water has both a hydrogen bond and a polar covalent bond. Hydrogen

Water Molecule Structure For Kids
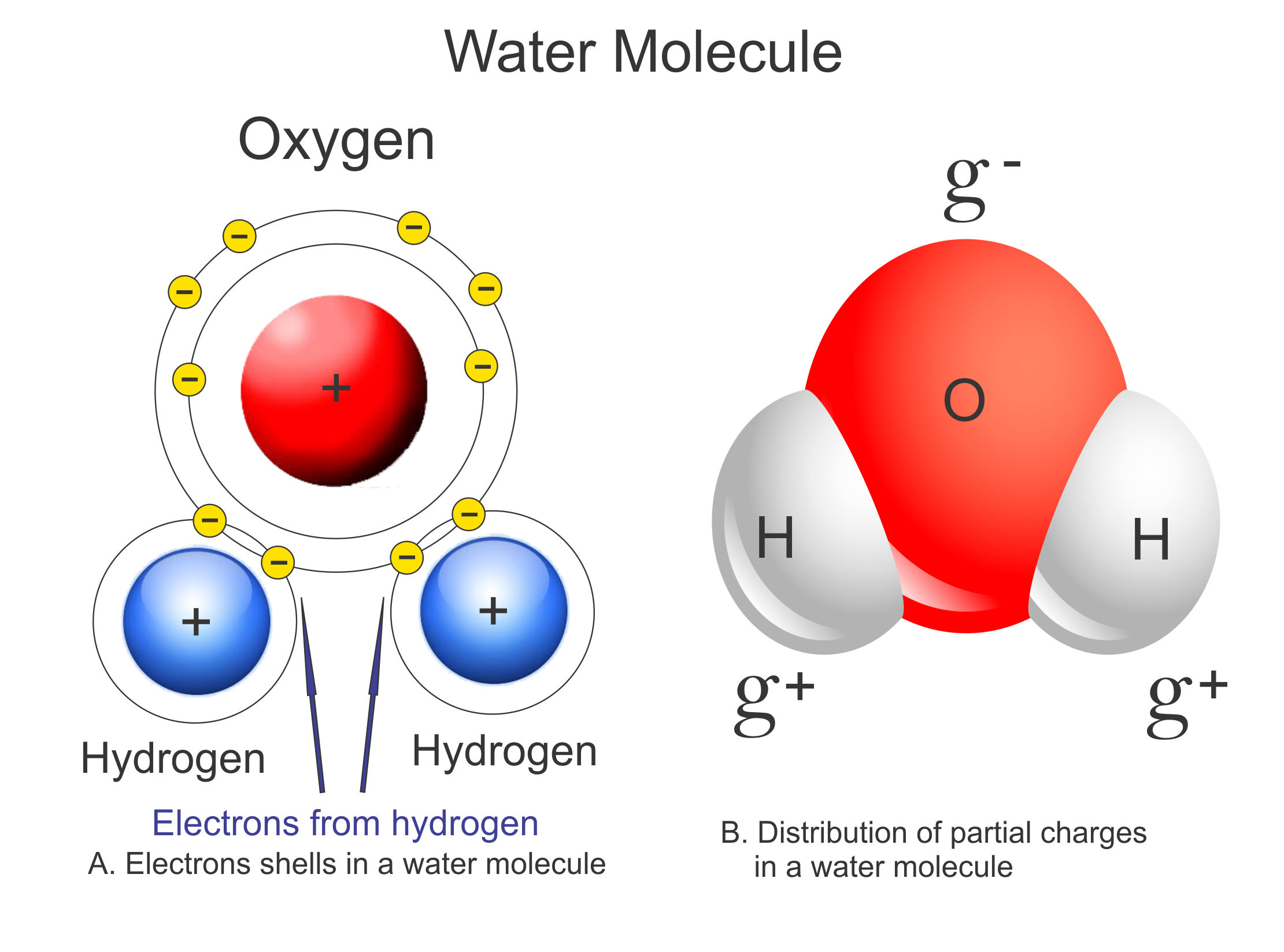
Polar Water Molecule Diagram
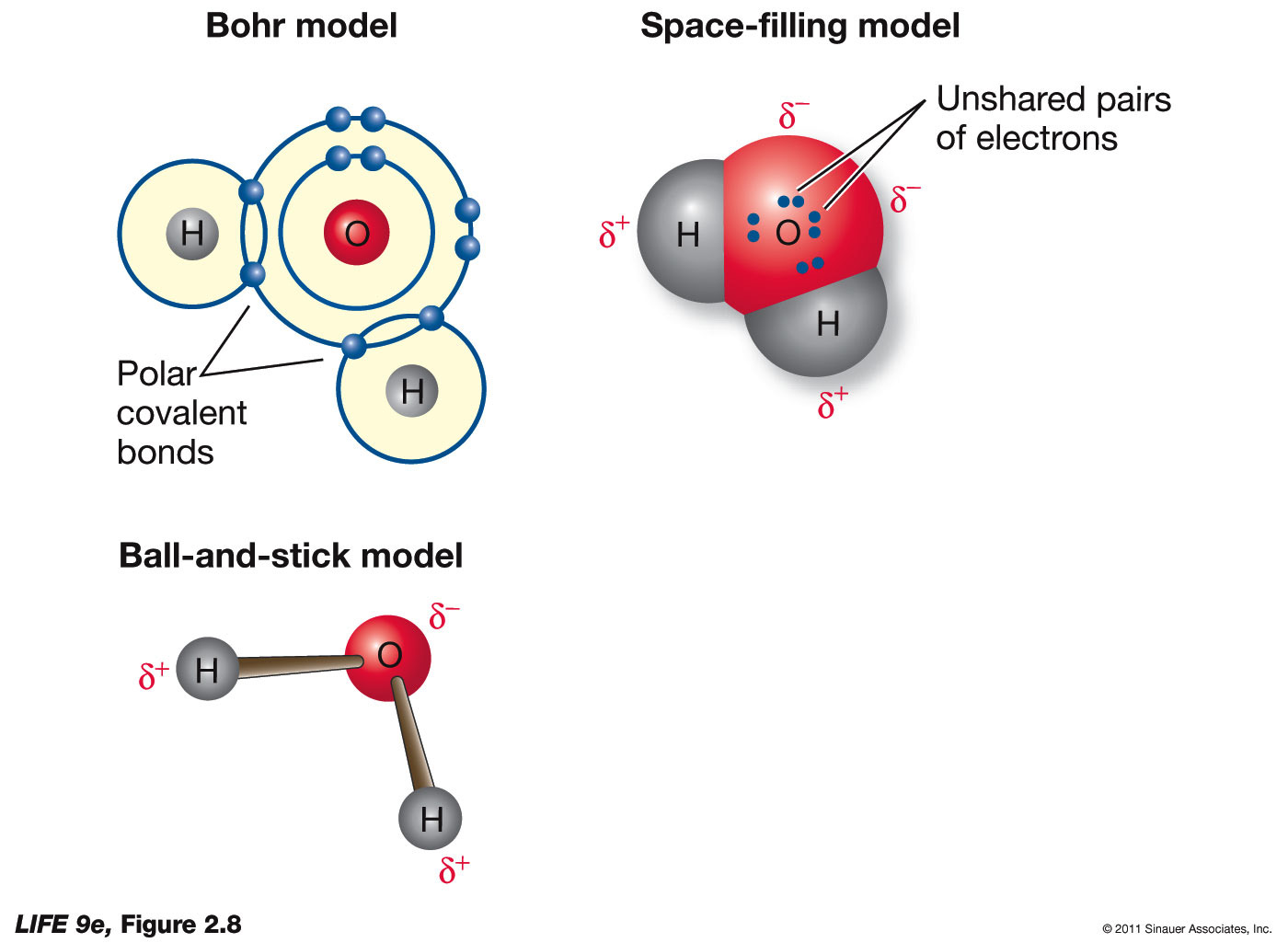
The BioLogs CAPE 1 Water Introduction to it's BIOchemistry
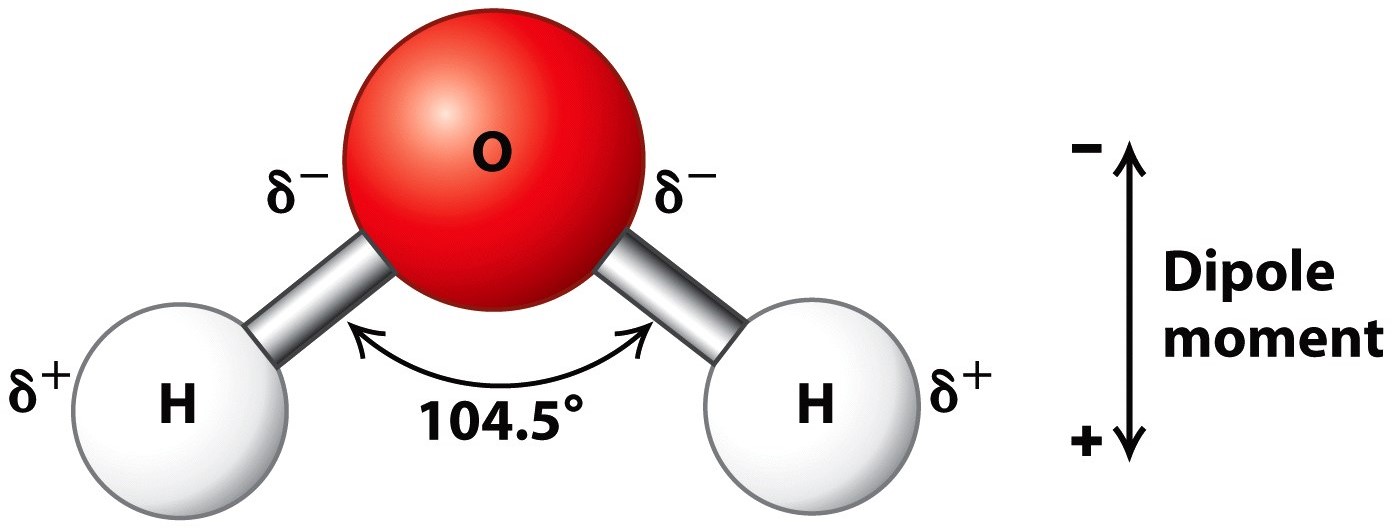
Describe the Structure of a Water Molecule

Types of Atoms Science at Your Doorstep
Solved Label the following diagram of water molecules,

The Structure and Properties of Water / Introduction to Chemistry

illustration of biochemistry, Water molecule consists of two hydrogen
Web Element That Has A Slight Negative Charge When Sharing Electrons Within A Water Molecule.
Video Answer Solved By Verified Expert Video By Farhan Anwar University Of Arizona | Answered On 02/28/2023 Video Answers To Similar Questions
Because The Water Molecule Has An H — O — H Bond Angle Of 105°, The Molecule As A Whole Is Polar.
Web Draw A Sketch Of Two Water Molecules Connected By A Hydrogen Bond.
Related Post: