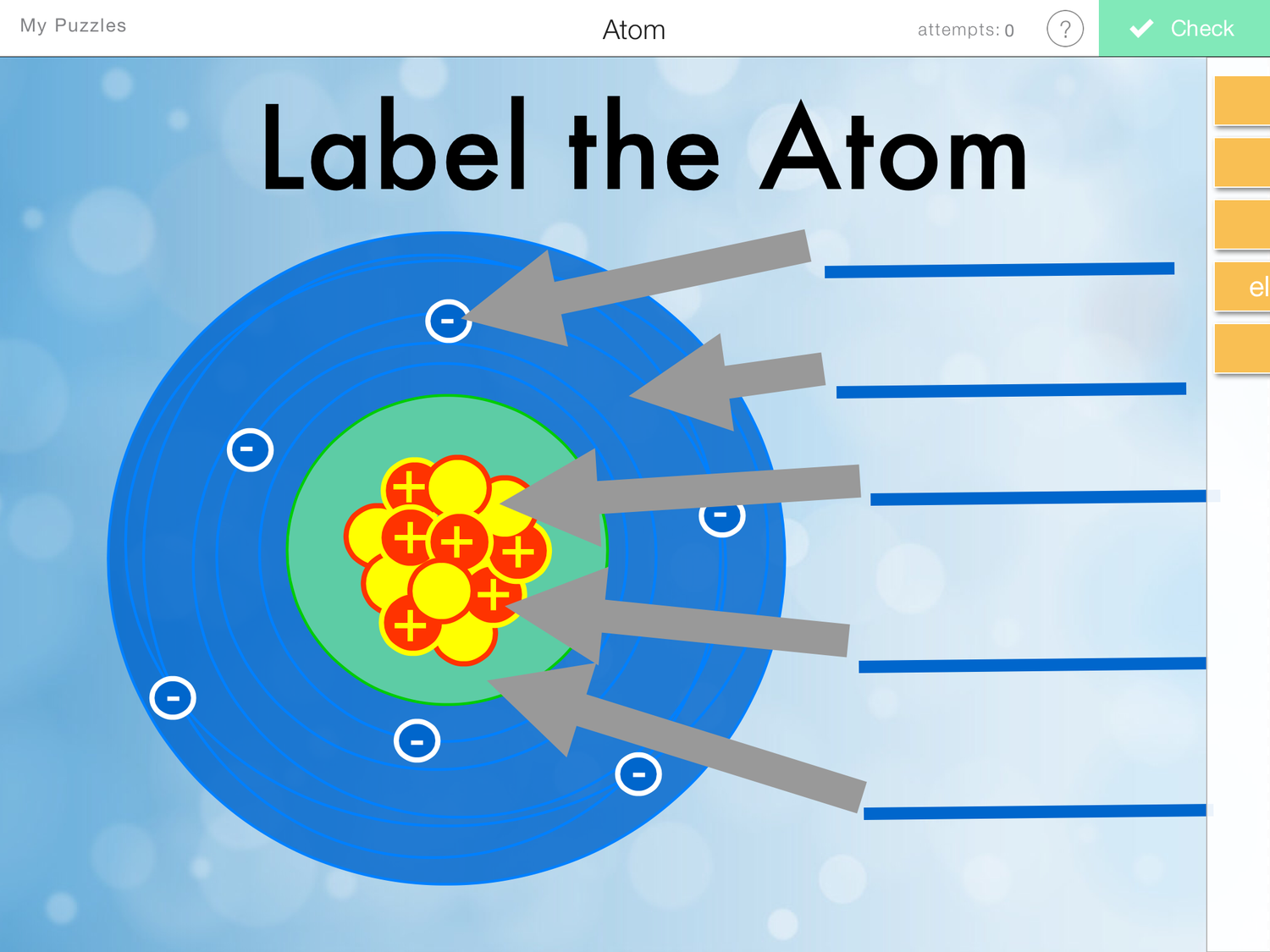
Draw And Label The Parts Of An Atom
Draw And Label The Parts Of An Atom - Web this is a list of the basic characteristics of atoms: These are the parts of the atom. Draw 3 electrons in the 2nd ndenergy level and label with their charge. Web every element is unique and has an atomic number. What does the mass of an atom consist of? For each electron shell atom diagram, the element symbol is listed in the nucleus. Are all atoms the same size? Web if you want (or need) to draw a model of an atom, we'll show you how! The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. Download complete chapter notes of structure of atom download now How is the atomic number of an atom defined? Find out how to draw a diagram of an atom with help from an artist. 1) electrons, 2) protons, and neutrons. Web with the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Atoms how atoms can be seen. Atoms are the smallest particle of matter than cannot be further subdivided using chemical means. The nucleus, the center of atom contain proton and neutron, and the outer portion of the atom holds electrons in its orbit around the nucleus [1]. This makes the nucleus positively charged. These are the parts of the atom. Atoms contain up of 3 basic. Web part of the series: Draw 2 electrons in the 1st energy level and label with their charge. That number tells you the number of protons in every atom of the element. These are the parts of the atom. Web what are the parts of an atom? Web here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The center of an atom is the nucleus that contain protons and neutrons. Atoms how atoms can be seen. Draw 2 electrons in the 1st energy level and label with their charge. Web particle in an atom with no charge. Are all atoms the same size? Atoms contain up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). Neutrons and protons are found at the center of the atom within a dense region called the nucleus. Web here are electron shell atom diagrams for the elements, ordered by increasing. Are all atoms the same size? Web with the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. Web if you want (or need) to draw a model of an atom, we'll show you how! The. The negatively charged particles called electrons revolve around the centre of the nucleus. That number tells you the number of protons in every atom of the element. Label them with their charge. Web atoms contain protons, neutrons and electrons. Draw two electrons in the first energy level and label them with their charge. That number tells you the number of protons in every atom of the element. Web every element is unique and has an atomic number. The electrons are arranged in shells around the nucleus. For simplicity, we will use the amu. Web particle in an atom with no charge. Have you ever heard about. Web sketch an atom draw 5 protons in the nucleus and label with the charge. Web figure 3.3.2 3.3. Find out how to draw a diagram of an atom with help from an artist. For simplicity, we will use the amu. Web particle in an atom with no charge. Elements, such as helium, depicted here, are made up of atoms. You must draw a diagram of an atom in a very specific way for maximum authenticity. Web if you want (or need) to draw a model of an atom, we'll show you how! How is the atomic number of an atom. These are the parts of the atom. Each of these parts has an associated charge, with protons carrying a. Web if you want (or need) to draw a model of an atom, we'll show you how! The atom is the basic building block of matter. The electron shells are shown, moving outward from the nucleus. All atoms except hydrogen contain three basic subatomic particles: They do consist of parts, which include protons, neutrons, and electrons, but an atom is a basic chemical building block of matter. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass number. What does the mass of an atom consist of? Draw five protons in the nucleus of the atom. Web the three main parts of an atom are protons, neutrons, and electrons. Atoms contain up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). Web every element is unique and has an atomic number. Draw six neutrons in the nucleus of the atom. Web with the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. Neutrons and protons are found at the center of the atom within a dense region called the nucleus.
Parts of An Atom Diagram Quizlet
Label Parts of an Atom — Learning in Hand with Tony Vincent
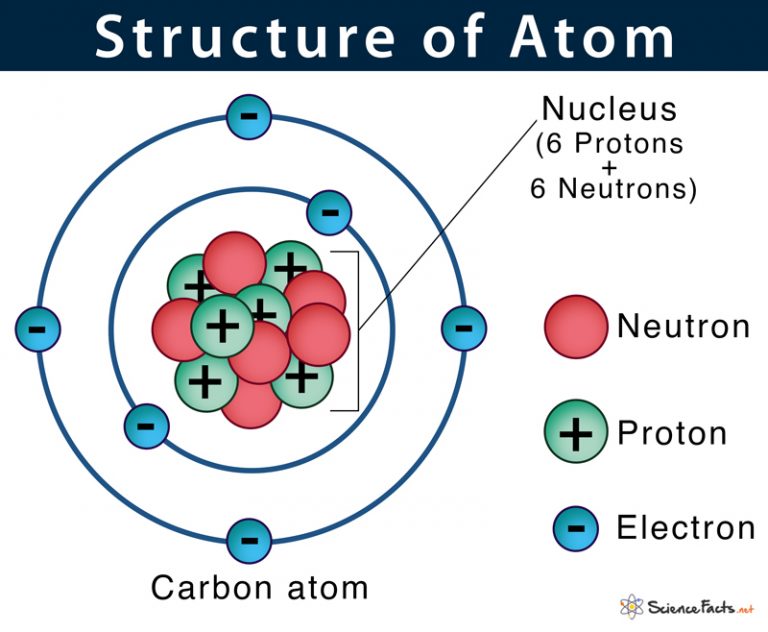
Atom Definition, Structure & Parts with Labeled Diagram

Atom Definition, Structure & Parts with Labeled Diagram
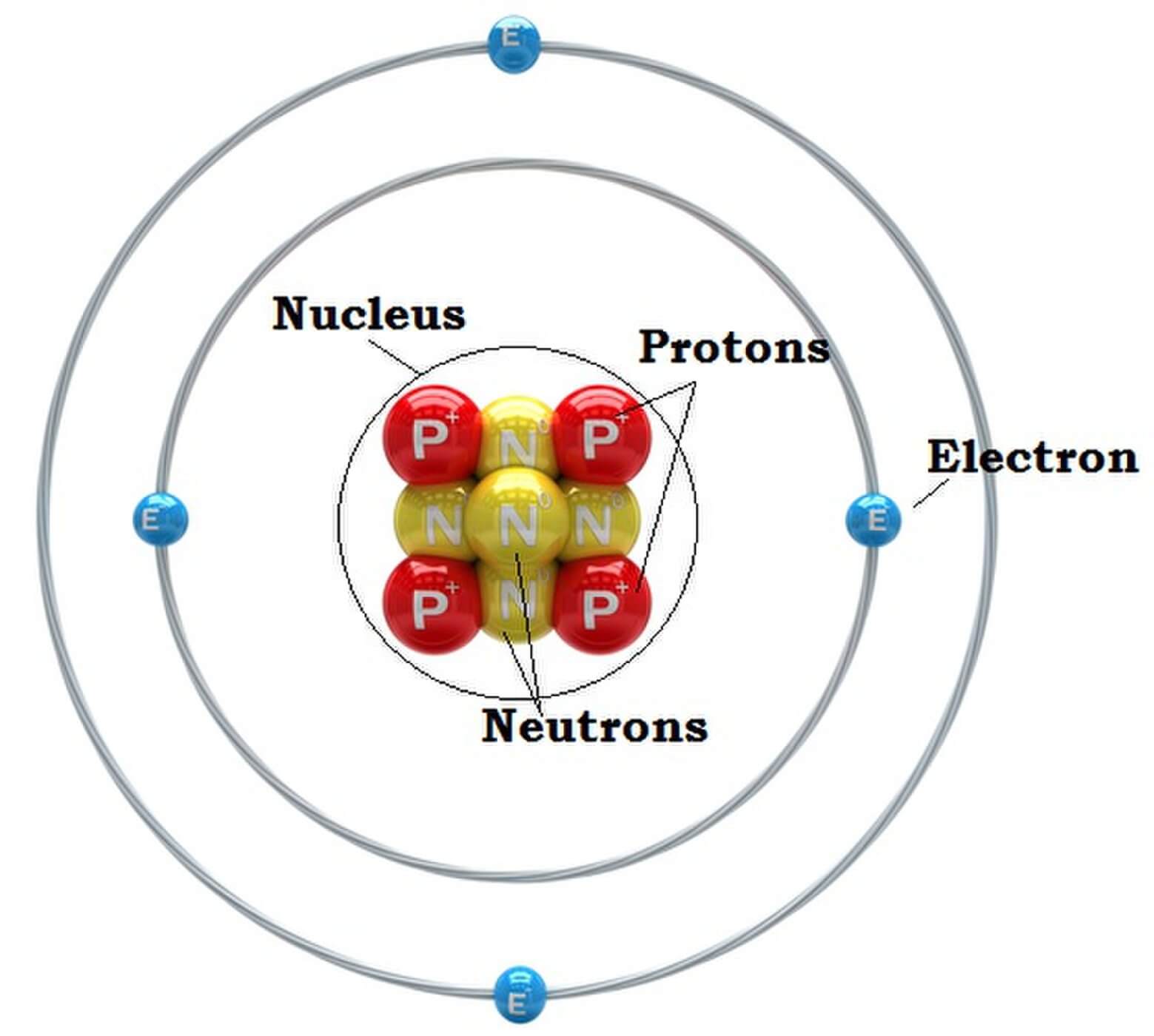
What is an Atom? Definitions & Examples Let us learn Basics News Bugz

Structure Of An Atom Class 9 Science Notes Leverage Edu

Learn the Parts of an Atom
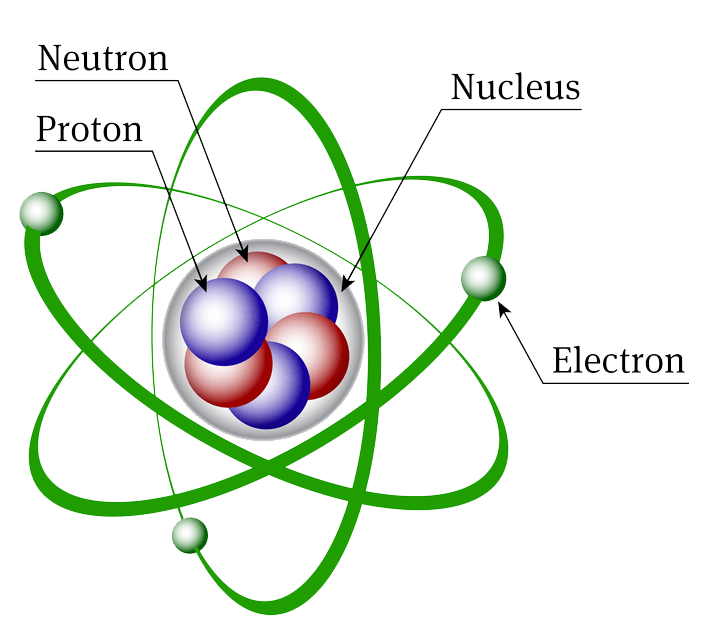
Atomic Structure Biochemistry
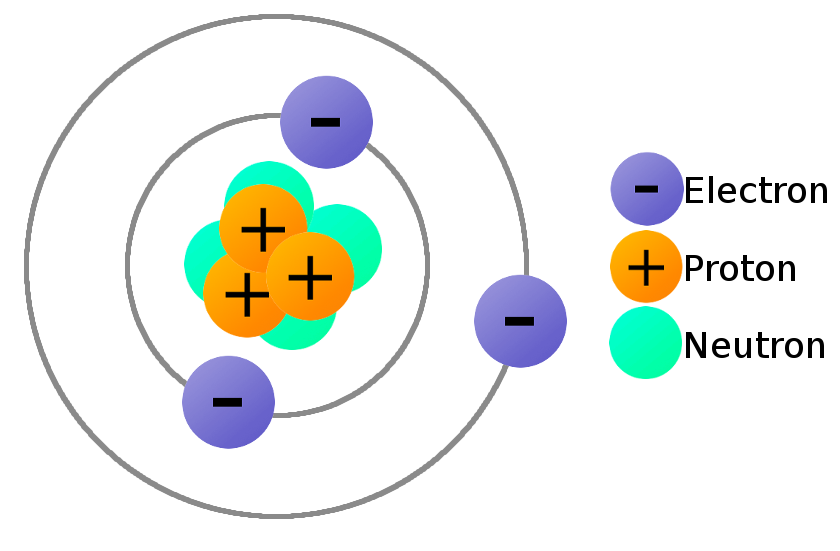
Structure of the Atom Unit G Review
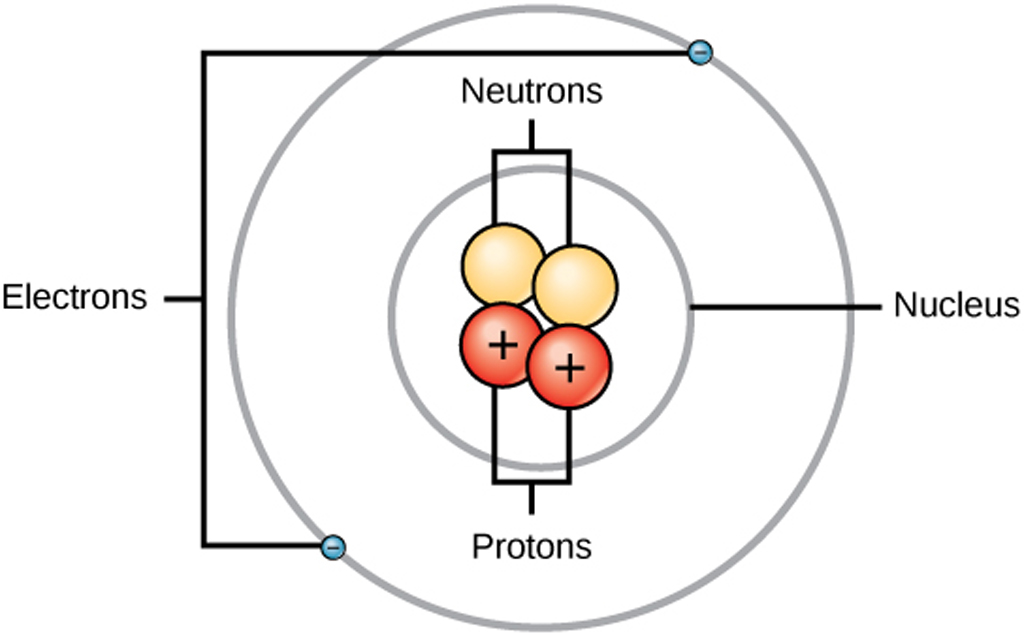
Structure of an Atom Structure & Use of Electron & Proton in Electronics
How Is The Atomic Number Of An Atom Defined?
That Number Tells You The Number Of Protons In Every Atom Of The Element.
Atoms How Atoms Can Be Seen.
The Electrons Are Arranged In Shells Around The Nucleus.
Related Post: