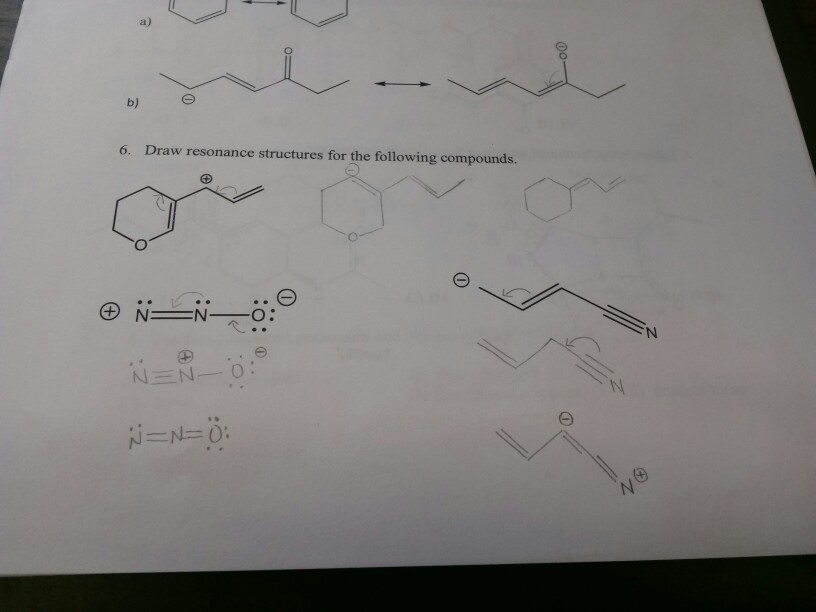
Draw Resonance Structures For The Following Compound
Draw Resonance Structures For The Following Compound - They must have the same number of electrons. Include only structures where a atoms (except h) have octets. In many cases, a single lewis structure fails to explain the bonding in a molecule/polyatomic ion due to the presence of partial charges and fractional bonds in it. Web resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Modify the second structure given to draw the new resonance structure. • you do not have to explicitly draw h. (ii) c h 2 = c h − c h = c h 2 (iii) c h 2 = c h − c | h = o solution verified by toppr (i) c h 2 = c h − c i ↔ c h 2 − c h = c i + (ii) c h 2 = c h − c h = c h 2 ↔ c h 2 − c h = c h = c h 2 ↔ c h = c h − c h 2 + (iii) c h 2 = c h c h o ↔ c h 2 − c h = c h o There is no change in hybridization between the structures. Determine the formal charge on each atom in each of the resonance structures:(a) o3(b) so. Some resonance structures are more favorable than others. Web the equivalent ressonance structures seem like the same but there are non equivalent ressonance strutures that occur when the delocalization of electrons is between qualitativity different bonds (they are different because they bond different atoms for instance a nitrogen and a carbon and two carbons) ( 6 votes) upvote flag show more. Which compound will have equally stable resonating. (d) c6h5cho (e) (f) view solution. Web step 1 2.48 the significant resonance structures for the compounds (a) and (b) are drawn below. (i) c h 2 = c h − c l. Indicate which would be the major contributor to the resonance hybrid. Include relevant formal charges in your structure. Only electrons move through the molecules. Web 1) for the following resonance structures please rank them in order of stability. H n oh ⊕ n oh h ⊕ n oh h ⊕ ⊕ o o ⊕ o ⊕ there are tw. Use the concept of resonance to explain structural features of molecules and ions. Draw a structure for benzene illustrating. Determine the formal charge on each atom in each of the resonance structures:(a) o3(b) so. In many cases, a single lewis structure fails to explain the bonding in a molecule/polyatomic ion due to the presence of partial charges and fractional bonds in it. Web art ii resonance draw all reasonable resonance structures for the following compound. Which of the following. Draw the resonance structures for the following compounds. Modify the second structure given to draw the new resonance structure. Indicate which would be the major contributor to the resonance hybrid. Web draw the resonance structures of molecules or ions that exhibit delocalization. A molecule that has several resonance structures is more stable than one with fewer. Web draw the resonance structures for the following compounds. In many cases, a single lewis structure fails to explain the bonding in a molecule/polyatomic ion due to the presence of partial charges and fractional bonds in it. The resonating structures of phenol are represented as: It compares and contrasts two or more possible lewis structures that can represent a particular. Step 1 first, add curved arrows) to show the resonance using the following pattern: A pi bond between two atoms of differing electronegativity. Determine the formal charge on each atom in each of the resonance structures:(a) o3(b) so. Web use resonance structures to describe the bonding in benzene. A molecule that has several resonance structures is more stable than one. Use the concept of resonance to explain structural features of molecules and ions. They must have the same number of electrons. Define and explain the differences between the following terms. Draw both resonance structures for the radical produced by reaction of the compound with a bromine atom. Web draw the resonance structures for the following compounds. Web practise questions on resonance structures. Indicate which would be the major contributor to the resonance hybrid. Equivalent lewis structures are called resonance forms. Include relevant formal charges in your structure. (b) the structure of c6h5no2 is: Indicate which would be the major contributor to the resonance hybrid. Equivalent lewis structures are called resonance forms. Web resonance is a mental exercise within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Web use resonance structures to describe the bonding in benzene. Determine the relative stability of resonance structures using a set of. The resonating structures of phenol are represented as: Web science chemistry chemistry questions and answers draw resonance structures for the following compound: 88% (16 ratings) view the full answer. Web draw the resonance structures for the following compounds. First, add curved arrow (s) to show the resonance using the following pattern: Step 1 first, add curved arrows) to show the resonance using the following pattern: Web step 1 2.48 the significant resonance structures for the compounds (a) and (b) are drawn below. (b) the structure of c6h5no2 is: Modify the second structure given to draw the new resonance structure. Only electrons move through the molecules. It compares and contrasts two or more possible lewis structures that can represent a particular molecule. Define and explain the differences between the following terms. They are used when there is more than one way to place double bonds and lone pairs on atoms. Draw both resonance structures for the radical produced by reaction of the compound with a bromine atom. (i) c h 2 = c h − c l. H n oh ⊕ n oh h ⊕ n oh h ⊕ ⊕ o o ⊕ o ⊕ there are tw.
OneClass Draw resonance structures for each of the following compounds
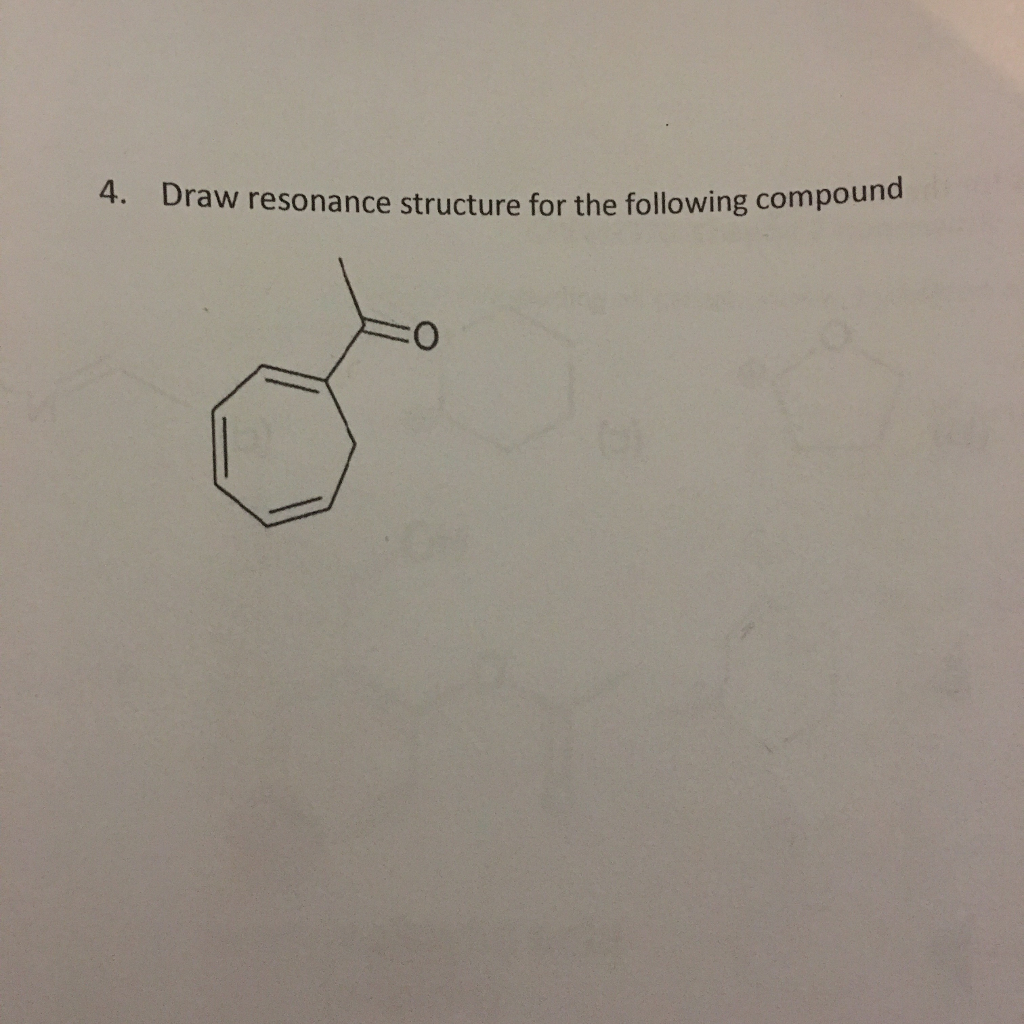
Solved Draw resonance structure for the following compund.
[Solved] . Draw resonance structures for the following compounds. Add
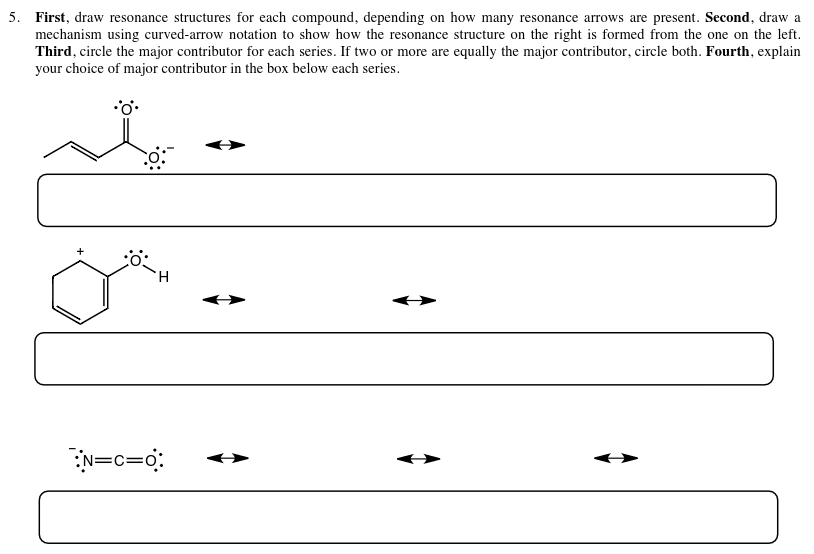
Solved First, draw resonance structures for each compound,
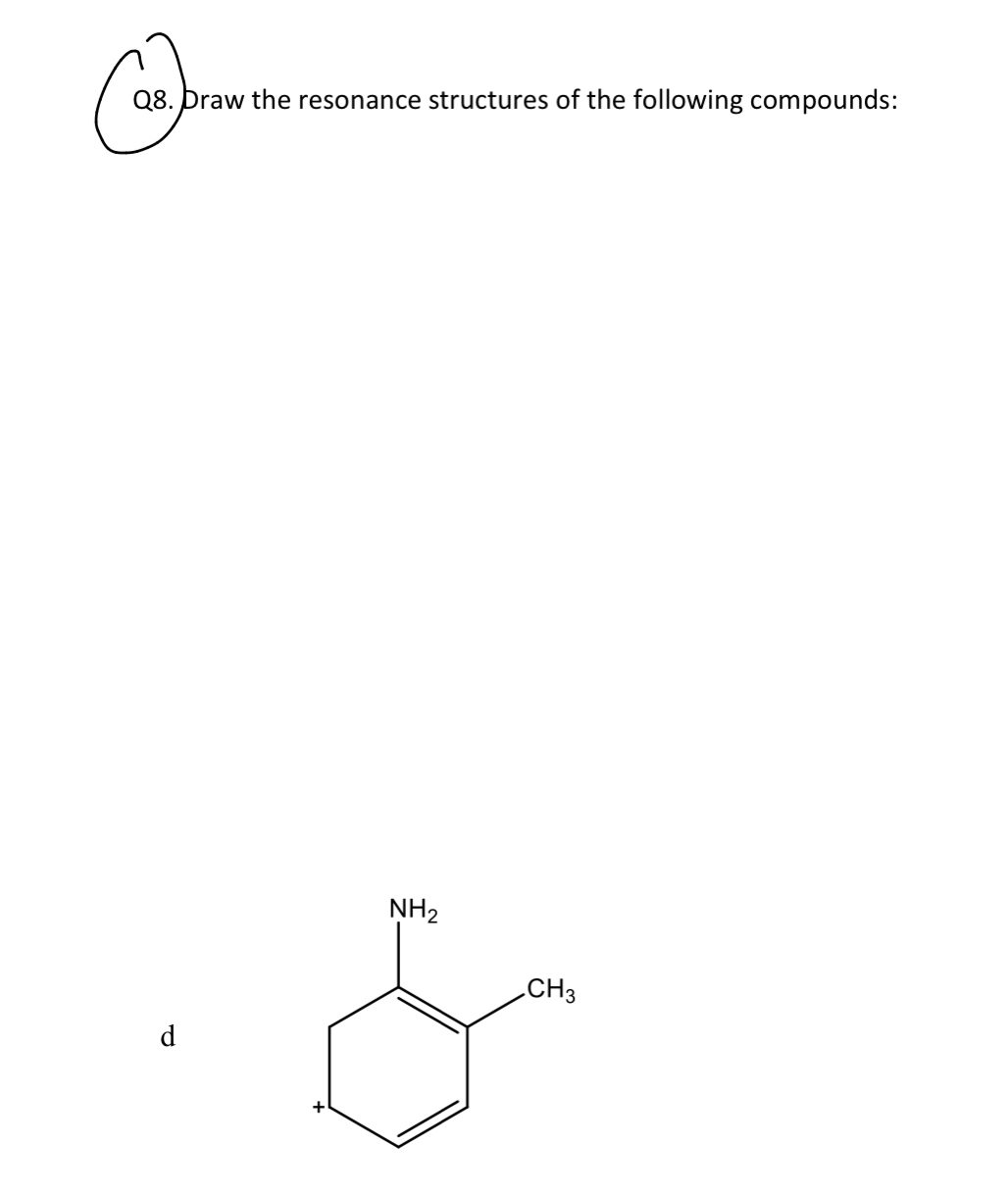
Answered Draw the resonance structures of the… bartleby
Solved a) 6. Draw resonance structures for the following
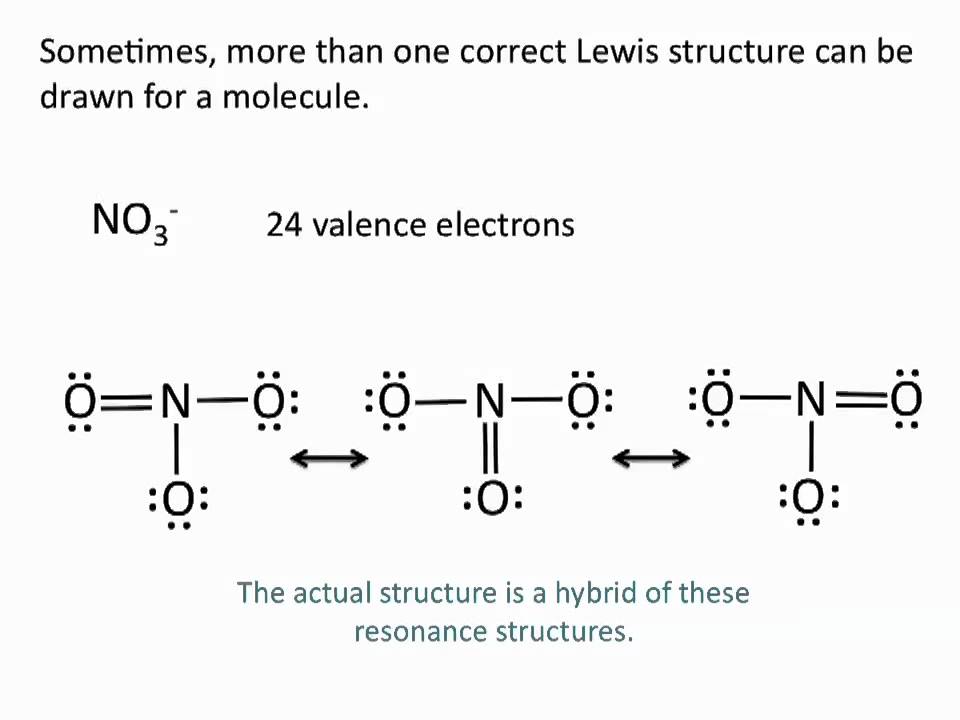
Drawing Lewis Structures Resonance Structures Chemistry Tutorial
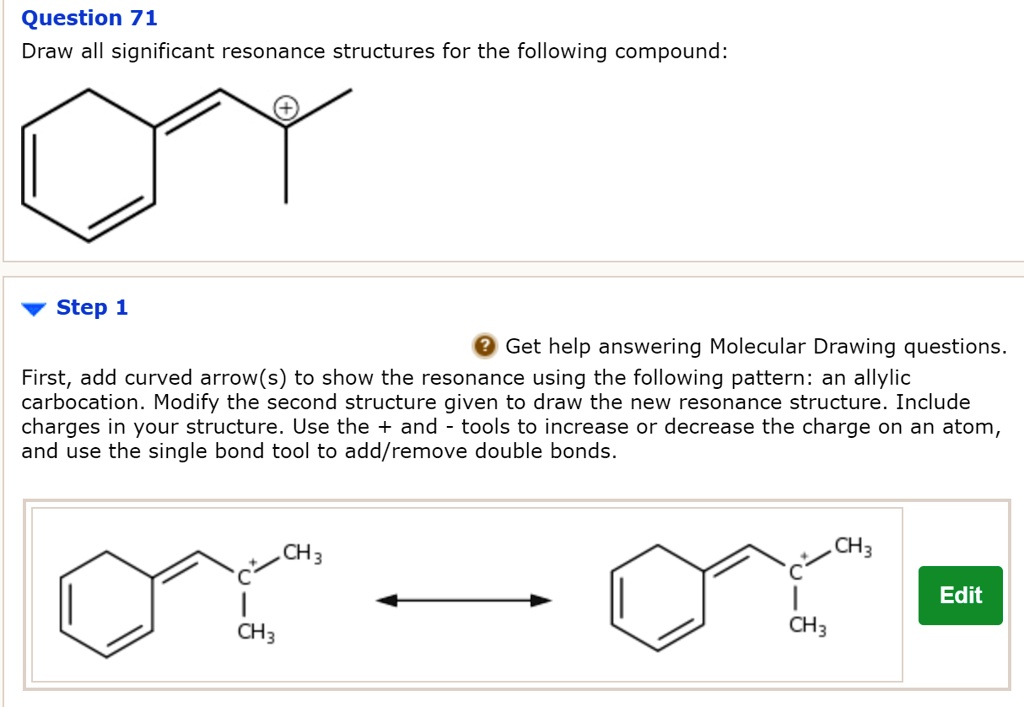
draw significant resonance structures for the following compound

Solved Draw significant resonance structures for the
[Solved] . 1. Draw resonance structures for the following compounds and
A Molecule That Has Several Resonance Structures Is More Stable Than One With Fewer.
Draw All Resonance Structures For The.
Web Draw Resonance Structures For The Following Compound:
Indicate Which Would Be The Major Contributor To The Resonance Hybrid.
Related Post: