Draw The Electron Configuration For A Neutral Atom Of Fluorine
Draw The Electron Configuration For A Neutral Atom Of Fluorine - All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Write the full electron configuration for a neutral fluorine atom. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n= 1 and the n= 2 shells are filled. 1s22s22p5 draw the lewis dot symbol for a neutral fluroine atom. When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s 2 2 p 6 electron configuration: The electron configurations and orbital diagrams of these four elements are: Web as an example, fluorine (f), has an atomic number of 9, meaning that a neutral fluorine atom has 9 electrons. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web electron configuration for fluorine electron configuration notation: A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n= 1 and the n= 2. Web the arrangement of electrons in fluorine in specific rules in different orbits and orbitals is called the electron configuration of fluorine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s. Select draw rings more erase f this problem has been solved! Web you can check it immediately in our valence electron calculator on the left or look at the position of a particular atom on the periodic table, which will help you to determine how many valence electrons a neutral atom of that element has. We add electrons to fill. Therefore, the number of electrons in neutral atom of fluorine is 9. Web chemistry groups what is the e. Web fluorine has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and 2 electrons pairs with one unpaired electron in the 2 p orbital. The elements of group 17 have seven electrons in. Web as an example, fluorine (f), has an atomic number of 9, meaning that a neutral fluorine atom has 9 electrons. Select draw rings more erase f this problem has been solved! 1s22s22p5 draw the lewis dot symbol for a neutral fluroine atom. Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5 and the orbital diagram is:. When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s 2 2 p 6 electron configuration: We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Similarly, fluorine has the electron configuration 1s 2 2s 2 2p. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web its electron configuration will be f: Web you can check it immediately in our valence electron calculator on the left or look at the position of a particular atom on the periodic table, which will help you to. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Draw the electron configuration for a neutral atom of fluorine. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. 1s. A slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). Web fluorine is the most electronegative element on the periodic table, which means that it is a very. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. Energy х write the electron configuration for a neutral atom of. Web you can check it immediately in our valence electron calculator on the left or look at the position of a particular atom on the periodic table, which will help you to determine how many valence electrons a neutral atom of that element has. The first 2 electrons are found in the first energy level, and the other 7 are. The answer is (3) a fluorine atom in an excited state right from the start, you should be able to look at the given electron configuration and say. Web write the full electron configuration for a neutral fluorine atom. How to write the electron configuration for fluorine The neutral atom chlorine (z=17), for instance has 17 electrons. Draw the electron configuration for a neutral atom of fluorine. Question what is the electron configuration of fluorine? Web one electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. The periodic table gives the following electron configuration: Because all the 2p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. When we reach neon, with z = 10, we have filled the 2 p subshell, giving a 1 s 2 2 s 2 2 p 6 electron configuration: 1s 2 2s 2 2p 3: Web the electronic configuration of anions is assigned by adding electrons according to aufbau principle.
fluorine orbital diagram Herbalned
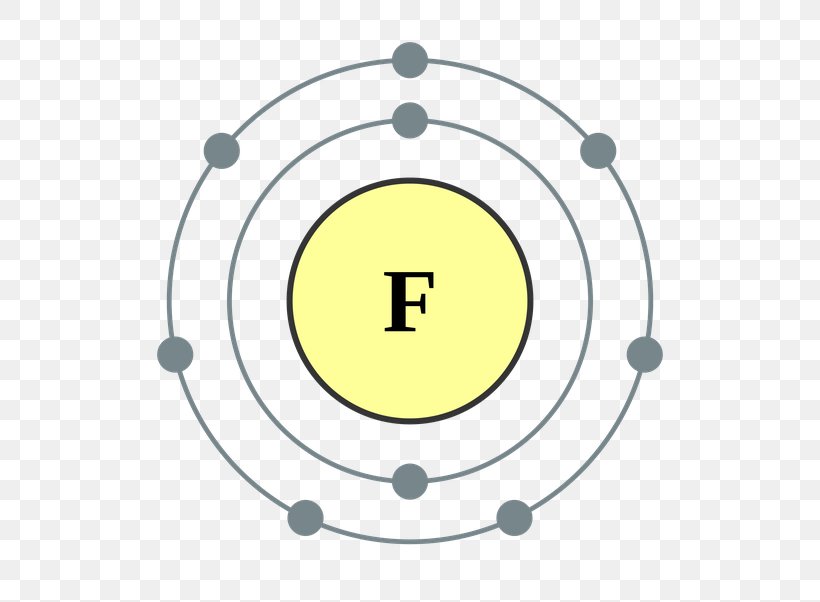
Periodic Table Fluorine Valence Electrons Periodic Table Timeline

Fluorine atom hires stock photography and images Alamy
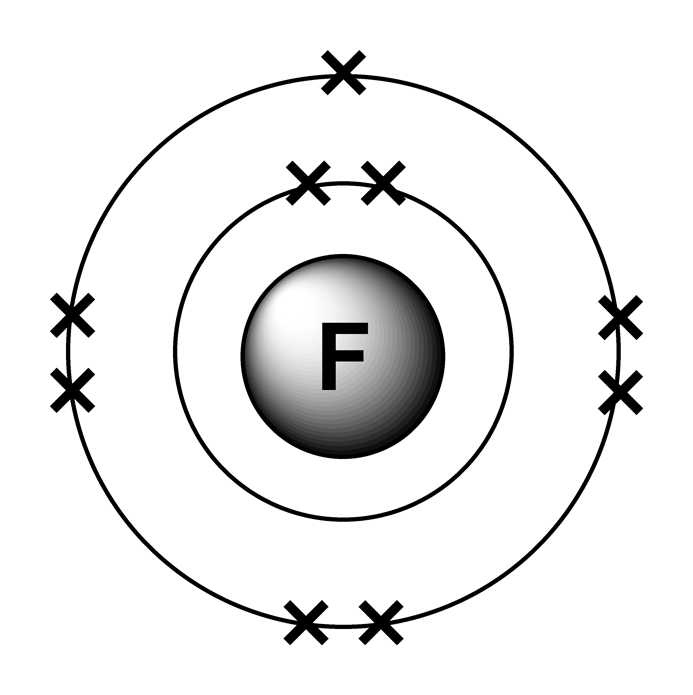
Electron configurations

Fluorine F (Element 9) of Periodic Table Elements Flash Cards

Fluorine Electron Configuration YouTube
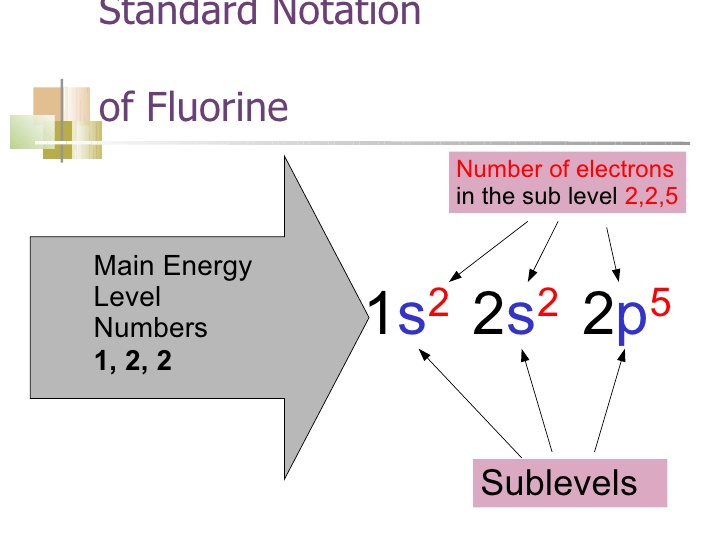
How To Find Electron Configuration For Fluorine Dynamic Periodic

Diagram representation element fluorine Royalty Free Vector

The Electron Configuration of Fluorine YouTube

How many valence electrons does fluorine(F) have?
Web As An Example, Fluorine (F), Has An Atomic Number Of 9, Meaning That A Neutral Fluorine Atom Has 9 Electrons.
Web The Arrangement Of Electrons In Fluorine In Specific Rules In Different Orbits And Orbitals Is Called The Electron Configuration Of Fluorine.
Which Means That The Electron Configuration Given To You Corresponds To A Fluorine Atom In An Excited State.
Web In This Case, 2+2+6+2+6+2+10+6+2+1= 39 And Z=39, So The Answer Is Correct.
Related Post: