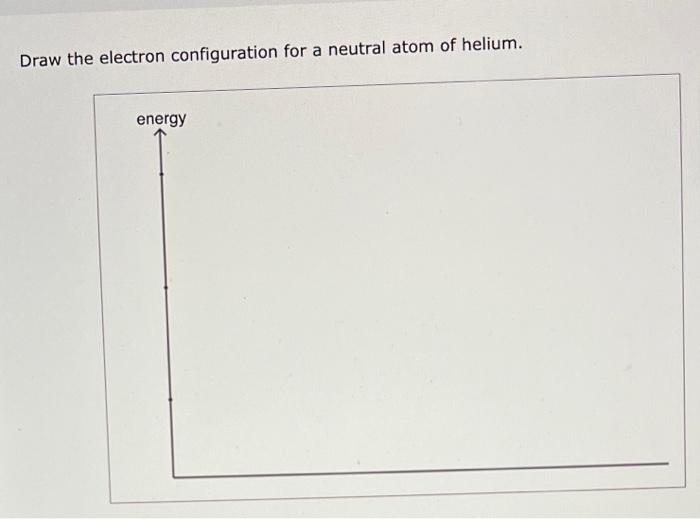
Draw The Electron Configuration For A Neutral Atom Of Helium.
Draw The Electron Configuration For A Neutral Atom Of Helium. - Helium has an atomic number of 2, which means it has 2 electrons. Web draw the electron configuration for a neutral atom of hellum. There was an issue loading this video view best match solution. Energy this problem has been solved! The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Answer the first energy level (n=1) can hold a maximum of 2 electrons. Video answer solved by verified expert oops! Electron configuration of beryllium (be) [he] 2s 2: Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. Draw the electron configuration for a neutral atom of helium. This problem has been solved! Web for each electron shell atom diagram, the element symbol is listed in the nucleus. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in. Following. We place one electron in the orbital that is lowest in energy, the 1 s orbital. Once it has grabbed a couple of electrons, a helium atom has been born. From the pauli exclusion principle , we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the. Web a neutral helium atom will thus have 2 electrons surrounding its nucleus. Draw the electron configuration for a neutral atom of helium. Following hydrogen is the noble gas helium, which has an atomic number of 2. Both of these electrons are located on the first energy level, in the only subshell, and consequently only orbital available to them. The. Web draw the electron configuration for a neutral atom of helium: Web electron configuration of hydrogen (h) 1s 1: There was an issue loading this video view best match solution. It means in case of neutral atom of helium. Following hydrogen is the noble gas helium, which has an atomic number of 2. Instant answer step 1/4 1. Therefore its electron configuration is, 1s2. It means in case of neutral atom of helium. ** except for helium, which has only two valence electrons. Web helium only has 2 electrons and therefore it has a configuration of 1s 2. This means that the electron configuration for helium has to account for only 2 electrons. We place one electron in the orbital that is lowest in energy, the 1 s orbital. Web step 1/2 the atomic number of helium is 2, which means it has 2 electrons. There was an issue loading this video view best match solution. From the. Following hydrogen is the noble gas helium, which has an atomic number of 2. The electron configuration for helium shows a full outer shell and is helium is therefore called a nobel gas. 1s, 2s, and then 2p. Therefore, the electron configuration for a neutral atom of helium is 1s2. Web the number of protons in an atom. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. Web helium only has 2 electrons and therefore it has a configuration of 1s 2. Both of these electrons are located on the first energy level, in the only subshell, and consequently only orbital available to them. Electron configuration of. This means it will not react with other atoms. This decay process for uranium is. Energy this problem has been solved! 2 electrons are present in s shell and is complete configuration in ground state. Web look at the periodic table and find an atomic number of oxygen, which is 8. 2 electrons are present in s shell and is complete configuration in ground state. 1s, 2s, and then 2p. Web chemistry chemistry questions and answers draw the electron configuration for a neutral atom of helium. Web electron configuration of hydrogen (h) 1s 1: Therefore, the number of electrons in neutral atom of helium is 2. We place one electron in the orbital that is lowest in energy, the 1 s orbital. The element atomic number and name are listed in the upper left. Electron configuration the arrangements of electrons above the last (closed shell) noble gas. Following hydrogen is the noble gas helium, which has an atomic number of 2. Draw the electron configuration for a neutral atom of helium. Energy 1 i x $ ? Web helium only has 2 electrons and therefore it has a configuration of 1s 2. The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Video answer solved by verified expert oops! Electron configuration of beryllium (be) [he] 2s 2: There was an issue loading this video view best match solution. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. We place one electron in the orbital that is lowest in energy, the 1 s orbital. Energy this problem has been solved! Web the number of protons in an atom. Therefore, the electron configuration for a neutral atom of helium is 1s2.:max_bytes(150000):strip_icc()/heliumatom-58b602983df78cdcd83d95c8.jpg)
Atoms Diagrams Electron Configurations of Elements

Helium Electron Configuration Electron configuration, Electrons
Helium Atom Drawing at GetDrawings Free download
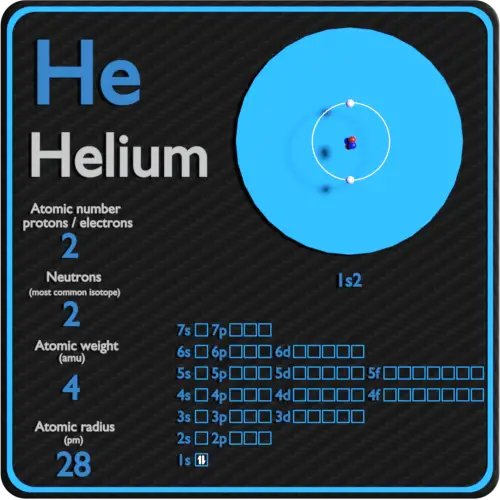
Helium Periodic Table and Atomic Properties
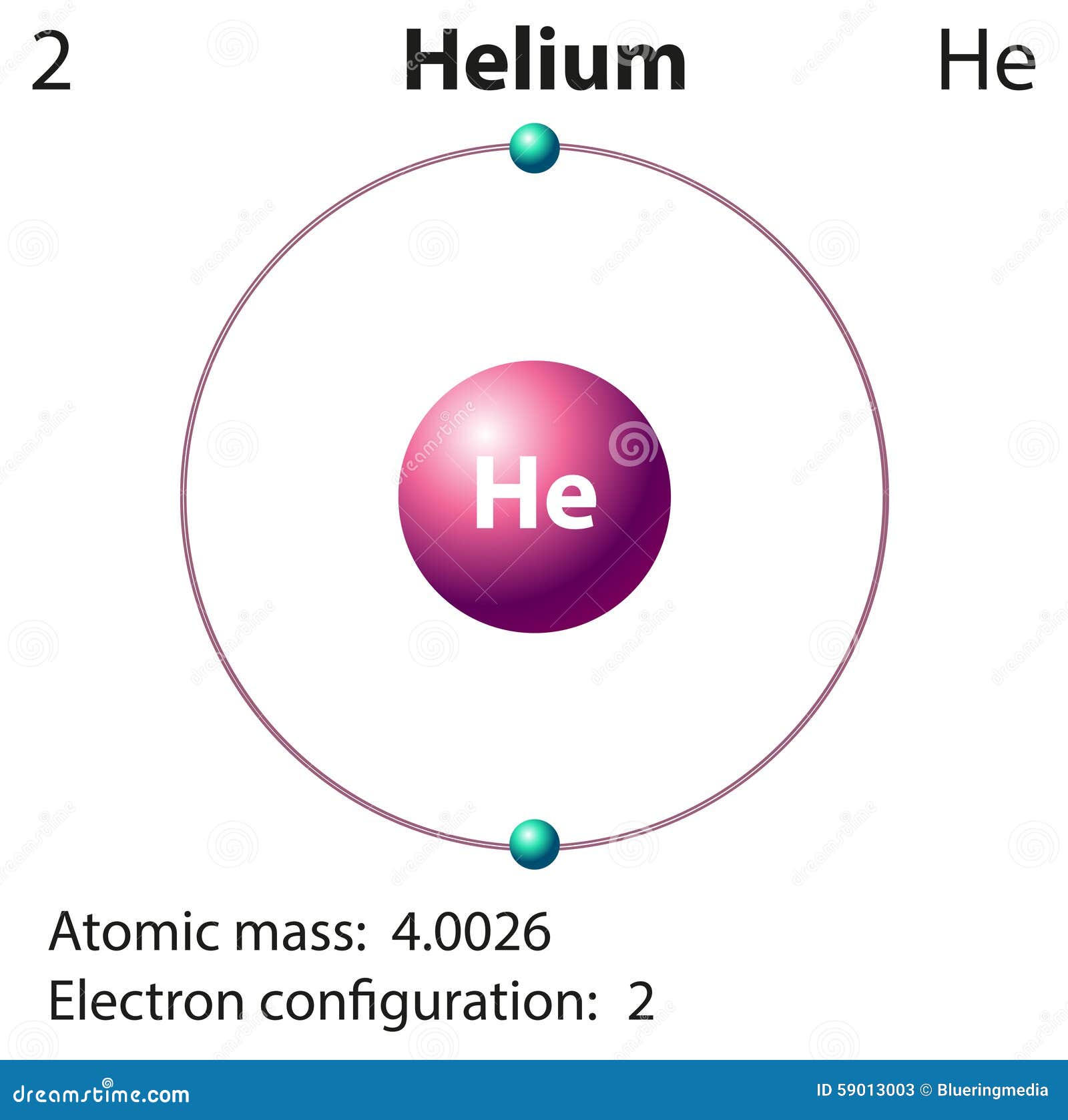
Helium Helium Electron Configuration

Electron Configuration Chart With Orbitals
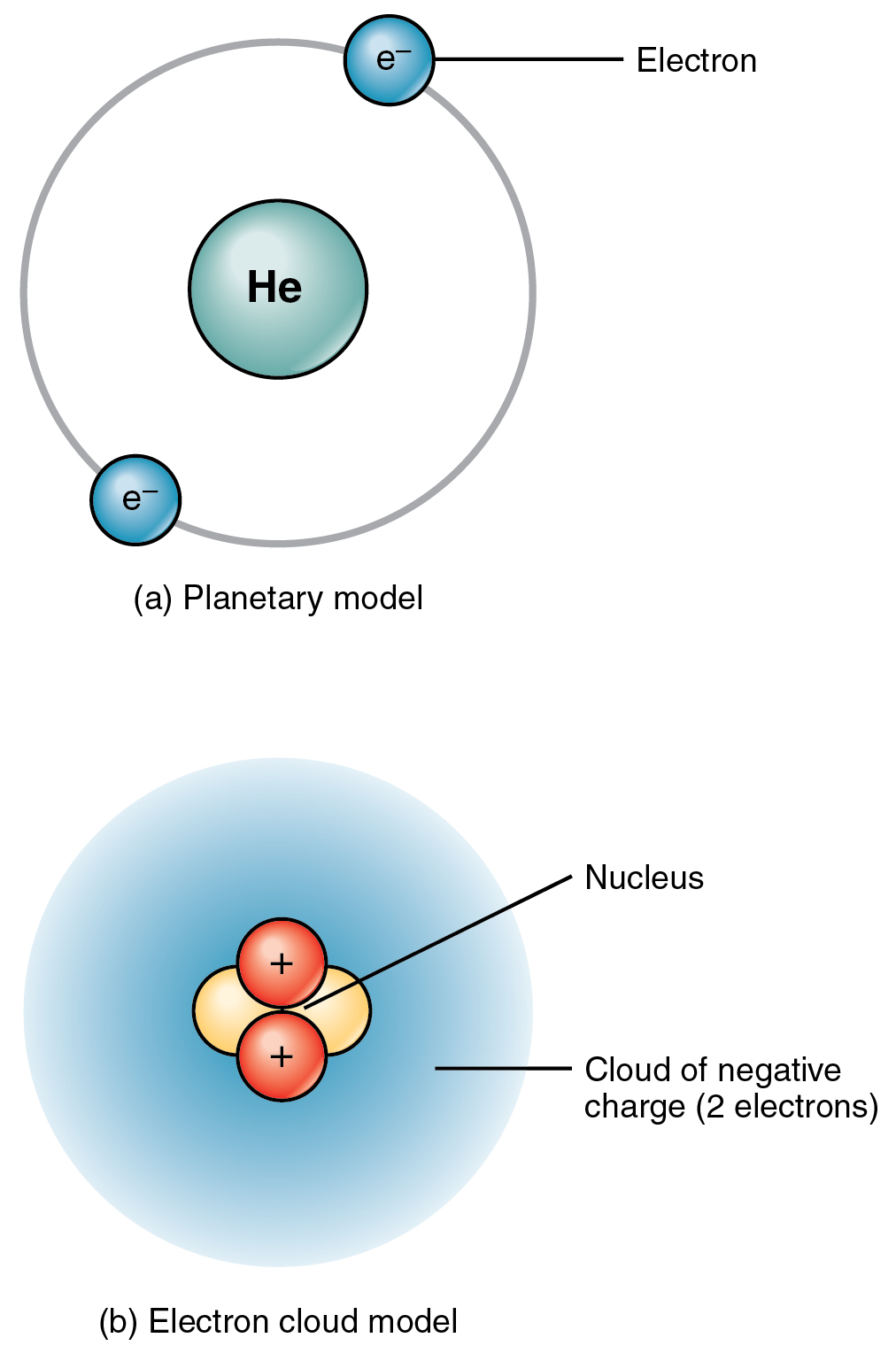
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
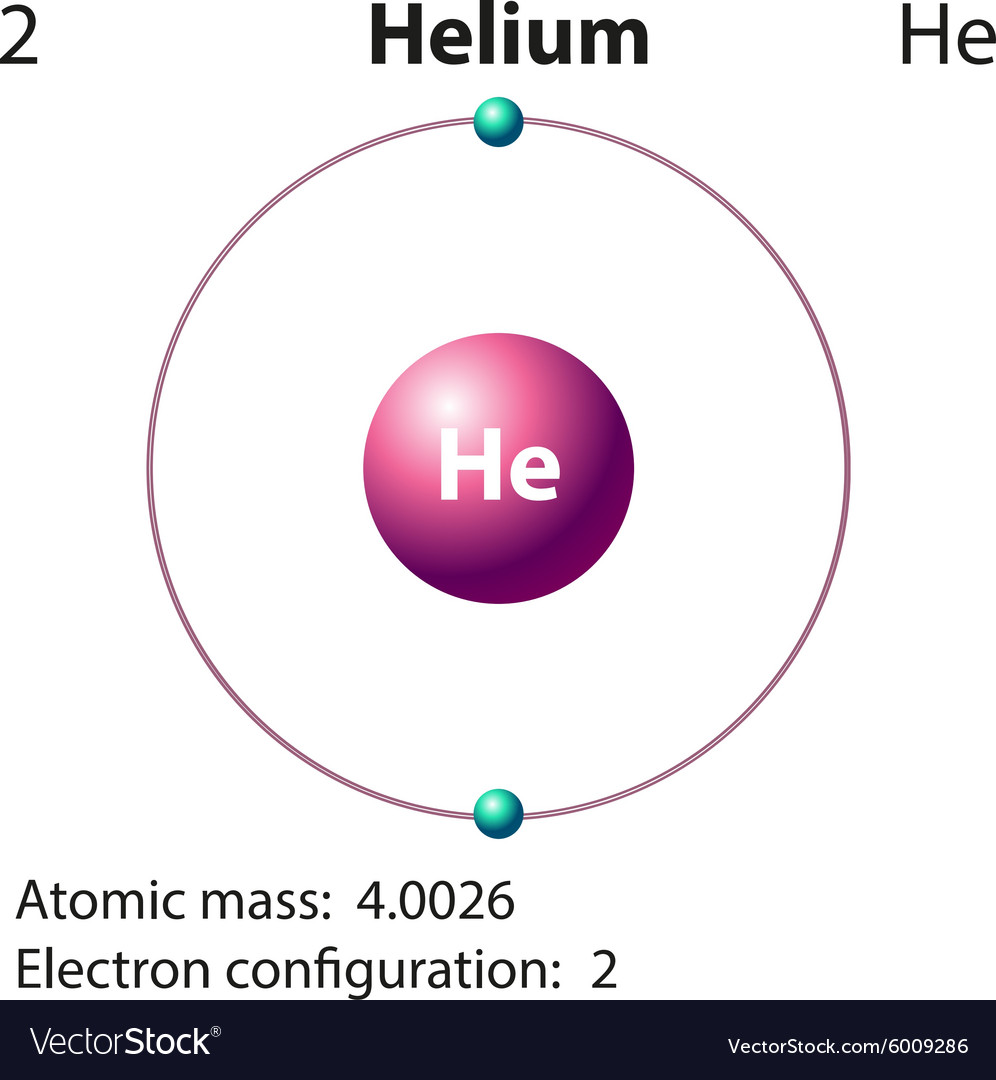
Diagram representation of the element helium Vector Image
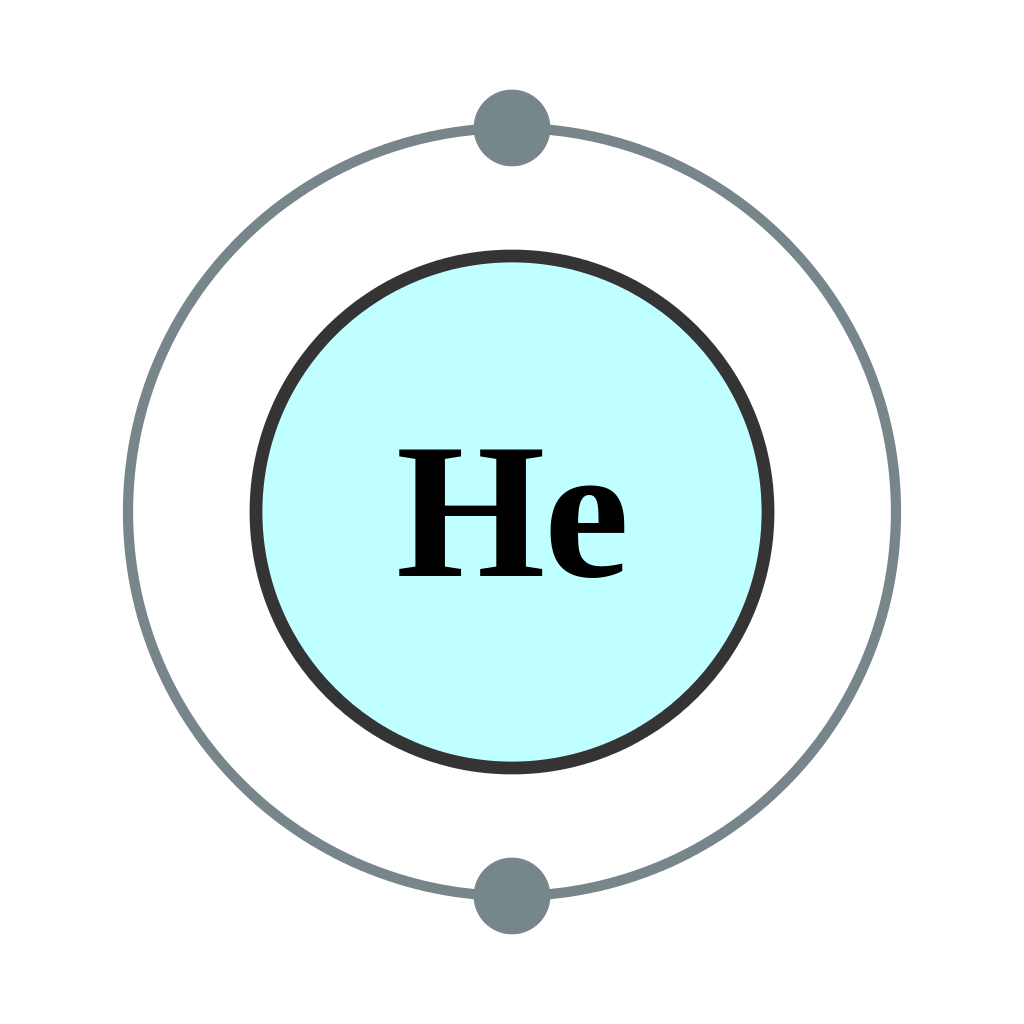
How To Find the Helium Electron Configuration (He)
Solved Draw the electron configuration for a neutral atom of
Because The 1S Orbital Is Full With 2 Electrons And Any Additional Electrons Would Go In A New Energy Level.
Electron Configuration Of Boron (B) [He] 2S 2 2P 1:
Write The Complete Electron Configuration Of Oxygen:
The Final Ring Or Shell Of Electrons Contains The Typical Number Of Valence Electrons For An Atom Of That Element.
Related Post: