Draw The Electron Configuration For A Neutral Atom Of Sulfur.
Draw The Electron Configuration For A Neutral Atom Of Sulfur. - As 1s only hold two electrons and the next two electrons for sulfur goes to the 2s orbital. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? • the atomic symbol of sulfur is s. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the. • the atomic mass of sulfur is 32.065. This is sometimes called the bohr, or the ‘solar system’, model. First, find electrons of sulfur atom. Which statement correctly describes the drawing of an atom's lewis symbol? So it is the same as neon, but with a full 3s2 subshell. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electron configuration of carbon (c) [he]. As 1s only hold two electrons and the next two electrons for sulfur goes to the 2s orbital. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. 1s 2 2s 2 2p 2: 100% (2 ratings) step 1. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) sulfur (s) atom electron. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? 1s 2 2s 2 2p 3: First, find electrons of sulfur atom. • the atomic symbol of sulfur is s. So it is the same as neon, but with a full 3s2 subshell. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into. So it is the same as neon, but with a full 3s2 subshell. Web what is the electron configuration of a neutral chlorine atom? Hence the s electron configuration is 1s 2 2s 2 2p. 1s 2 2s 2 2p 2: 1s 2 2s 2 2p 3: Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. But let's look, once again, at the selection rules for atomic orbitals. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Electron configuration of carbon. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that. The atomic number of the sulfur is 16. Ground state electron configuration of sulfur. Hence the s electron configuration is 1s 2 2s 2 2p. Web the electron configuration of sulfur is [ ne] 3s 2 3p 4 , if the electron arrangement is through orbitals. This is the electron configuration of helium; Web the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal. Locate the nearest noble gas preceding phosphorus in the periodic table. Hence the. The electron configuration for a sulfur atom is [ne]3s23p4 and its lewis symbol is shown in the figure below. This is sometimes called the bohr, or the ‘solar system’, model. The neutral atom chlorine (z=17), for instance has 17 electrons. Since the atomic number of sulfur is 16, the total electrons of sulfur are 16. Therefore, the number of electrons. Web there are six valence electrons in the outer shell of the sulfur. 100% (2 ratings) step 1. When we the electron configuration of sulfur the first two electrons go in the 1s orbital. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) sulfur (s) atom electron configuration (bohr model) Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. This is sometimes called the bohr, or the ‘solar system’, model. The electron configuration for a sulfur atom is [ne]3s23p4 and its lewis symbol is shown in the figure below. Web science chemistry chemistry questions and answers 1. Web • the electronic configuration of sulfur is [ne] 3s 2 3p 4. First, find electrons of sulfur atom. Which statement correctly describes the drawing of an atom's lewis symbol? 1s 2 2s 2 2p 3: We will use this information to draw the bohr model of sulfur atom. 1s 2 2s 2 2p 1: Therefore, the number of electrons in neutral atom of sulfur is 16.
Sulfur S (Element 16) of Periodic Table Elements FlashCards

Diagram representation of the element sulfur Vector Image
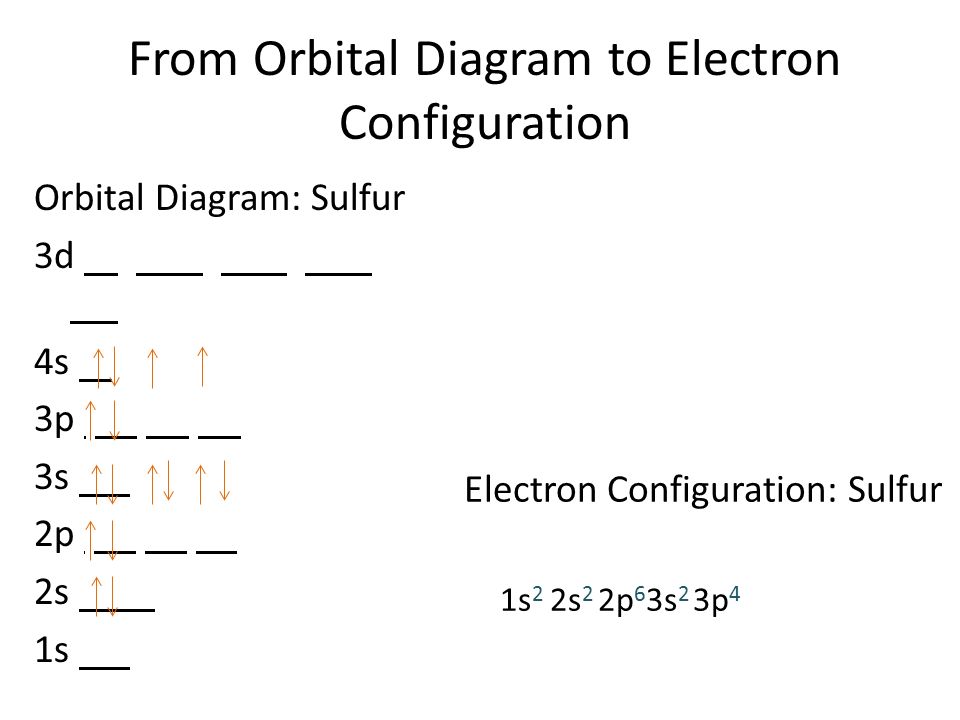
Sulfur Electron Configuration (S) with Orbital Diagram
/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atoms Diagrams Electron Configurations of Elements
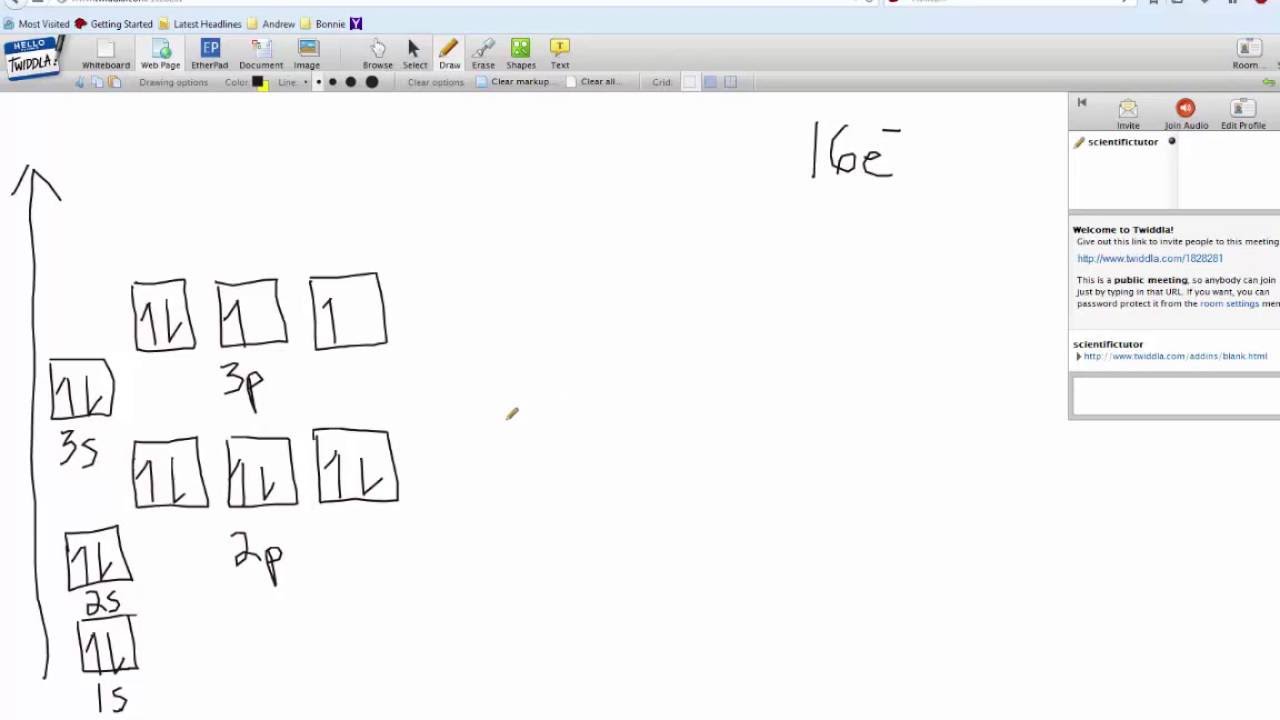
Electron Configuration Orbital Diagram Sulfur YouTube

The Orbital Diagram Shows the Valence Electrons of Sulfur
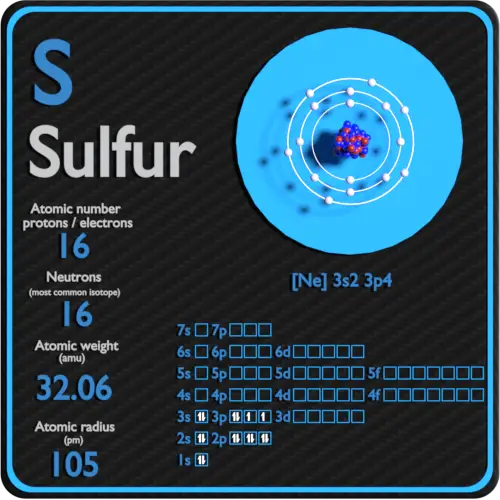
Sulfur Protons Neutrons Electrons Electron Configuration

WebElements Periodic Table " Sulfur " properties of free atoms Heading
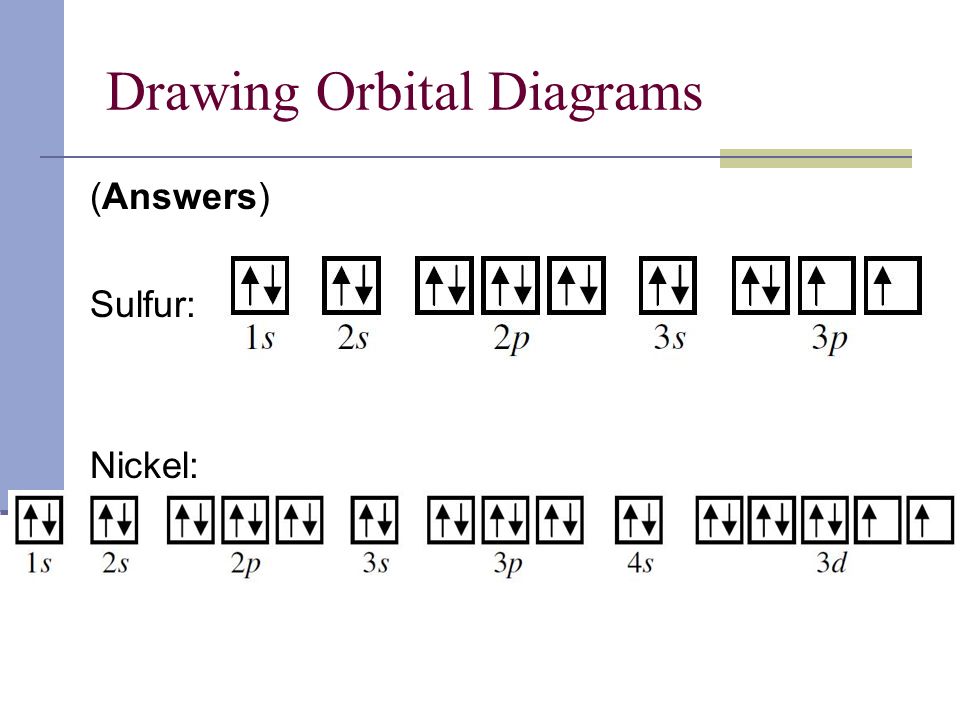
Orbital Box Diagram For Sulfur

Sulfur Atom Science Notes and Projects
Neutal Sulfur Atom Contains 16 Electrons.
This Is The Electron Configuration Of Helium;
Web The Electron Configuration Of Sulfur Is [ Ne] 3S 2 3P 4 , If The Electron Arrangement Is Through Orbitals.
1S 2 2S 2 2P 2:
Related Post: