Draw The Electron Dot Formula For The Element Sulfur
Draw The Electron Dot Formula For The Element Sulfur - The electrons are drawn in a clockwise manner starting on the right. This problem has been solved! Web the core electrons would be 1 s2 2 s2 2 p6 while the valence electrons would be in the third shell (or where n = 3). Include all of the valence electrons. Some types of rock can even completely dissolve in water ( figure below)! For sulfur there are six valence electrons in 3s and 3p shell. On the periodic table, 6 valence electrons for sulfur, 7 for fluorine but we have 4 fluorines; You can add the valence electrons by clicking on the or button and clicking the atom. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. These electrons are shown by dots around the atomic symbol. Lewis dot structure of sulfur atom sulfur ion lewis dot structure The most famous allotrope that exists from these elements is a diamond, which is an allotrope of carbon. Web water is an amazing molecule. The electrons are drawn in a clockwise manner starting on the right. The lewis symbol for sulfur will therefore be the symbol 's' surrounded by six dots meant to represent these six electrons. You can add the valence electrons by clicking on the or button and clicking the atom. Lewis dot structure of sulfur atom sulfur ion lewis dot structure Include all of the valence electrons. Include all of the valence electrons. Web only five elements are known to have allotropes: Regular additions of sulfur will ensure that the conversion process is continually occurring and continually providing the beneficial acidifying effect. We'll put the s, the least electronegative, at the center. Sulfur , which is located in group 6a, has 6 valence electrons. It has a very simple chemical formula, h 2. In alchemy, it could also represent evaporation, expansion, and dissolution. We'll put the fluorines around it, all four of them. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Therefore, chlorine has 7 valence electrons. The lewis electron dot diagram would look like the following: In terms of human body, it represented the soul. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Some types of rock can even completely dissolve in water ( figure below)! Web. In terms of human body, it represented the soul. You can add the valence electrons by clicking on the or button and clicking the atom. Water is remarkable in terms of all the things it can do. The formal charge on the sulfur atom is therefore 6 −(6 + 2 2) = −1.5 −(4 + 4 2) = −1 in. It has a very simple chemical formula, h 2 o. Therefore, chlorine has 7 valence electrons. Water is remarkable in terms of all the things it can do. You can add the valence electrons by clicking on the or button and clicking the atom. To eliminate sodium, an element essential to human health but harmful to plants, sulfur needs a. Web sulfur represents properties such as dryness, heat, and masculinity. I show you where sulfur is on the periodic table and how to determine how many valence electrons sulfur. In terms of the tria prima, sulfur was seen as the middling element connecting salt (high) and mercury (low). Web chemicals in the soil. The electrons are drawn in a clockwise. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The element sulfur (s) has six valence electrons in its outer shell. 100% (1 rating) transcribed image text: This problem has been solved!. Include all of the valence electrons. · s ····↙·↘···· in the case of sulfur , which is an element in the periodic table with atomic number 16, it has six valence electrons in its outermost shell. For sulfur there are six valence electrons in 3s and 3p shell. The lewis electron dot diagram would look like the following: 100% (1. The electrons are drawn in a clockwise manner starting on the right. Include all of the valence electrons. The lewis symbol for sulfur will therefore be the symbol 's' surrounded by six dots meant to represent these six electrons. Include all of the valence electrons. Some types of rock can even completely dissolve in water ( figure below)! Water is remarkable in terms of all the things it can do. Since fluorine is found in group 7a of the periodic table, it contains 7 valence electrons. Web sulfur represents properties such as dryness, heat, and masculinity. We'll put the fluorines around it, all four of them. It has a very simple chemical formula, h 2 o. In alchemy, it could also represent evaporation, expansion, and dissolution. For sulfur there are six valence electrons in 3s and 3p shell. The lewis electron dot diagram would look like the following: Regular additions of sulfur will ensure that the conversion process is continually occurring and continually providing the beneficial acidifying effect. Tin, phosphorus, sulfur, oxygen, and carbon. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:
Sulfur Atom Science Notes and Projects
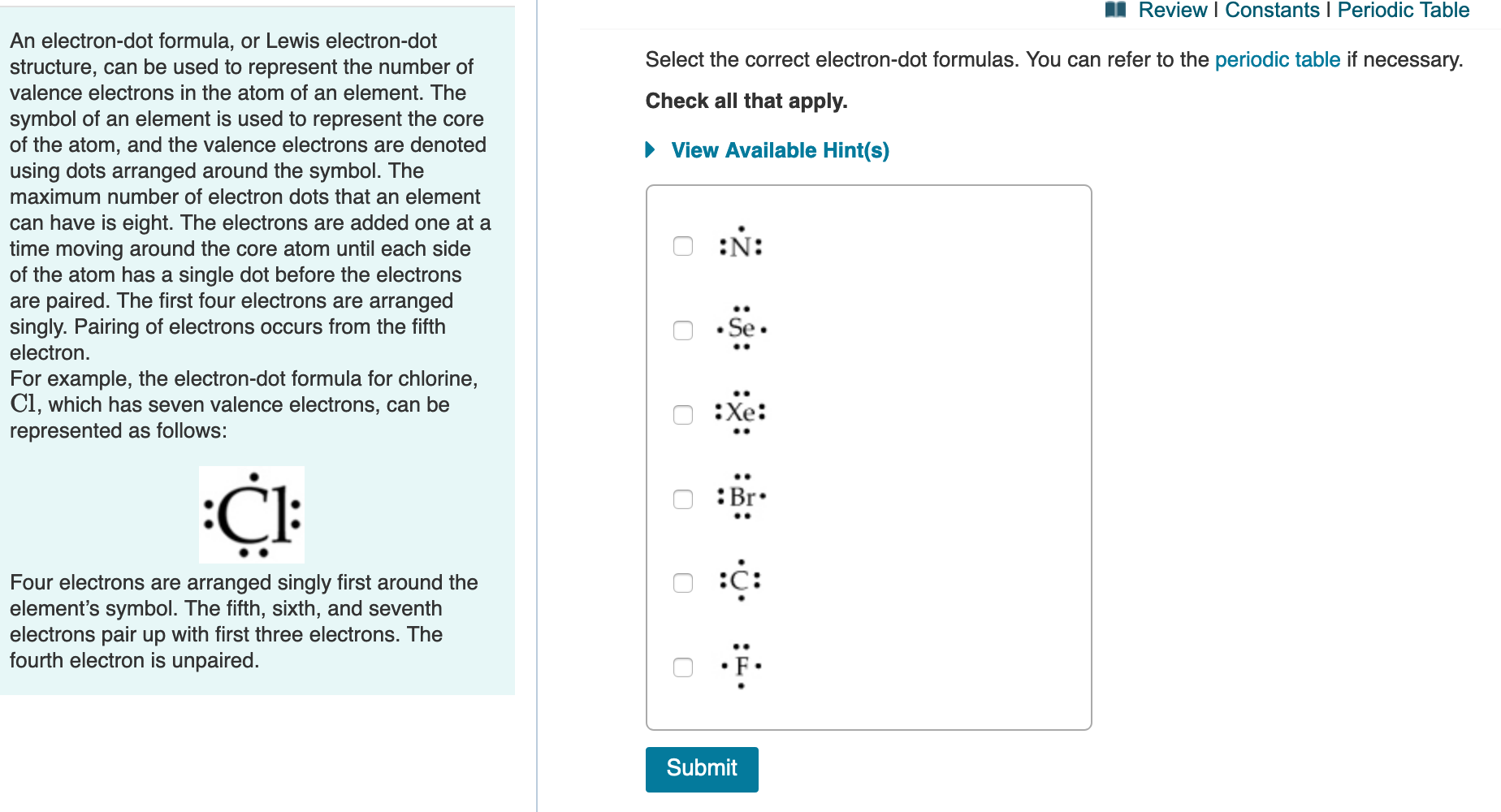
Solved Draw the electrondot formula for the element
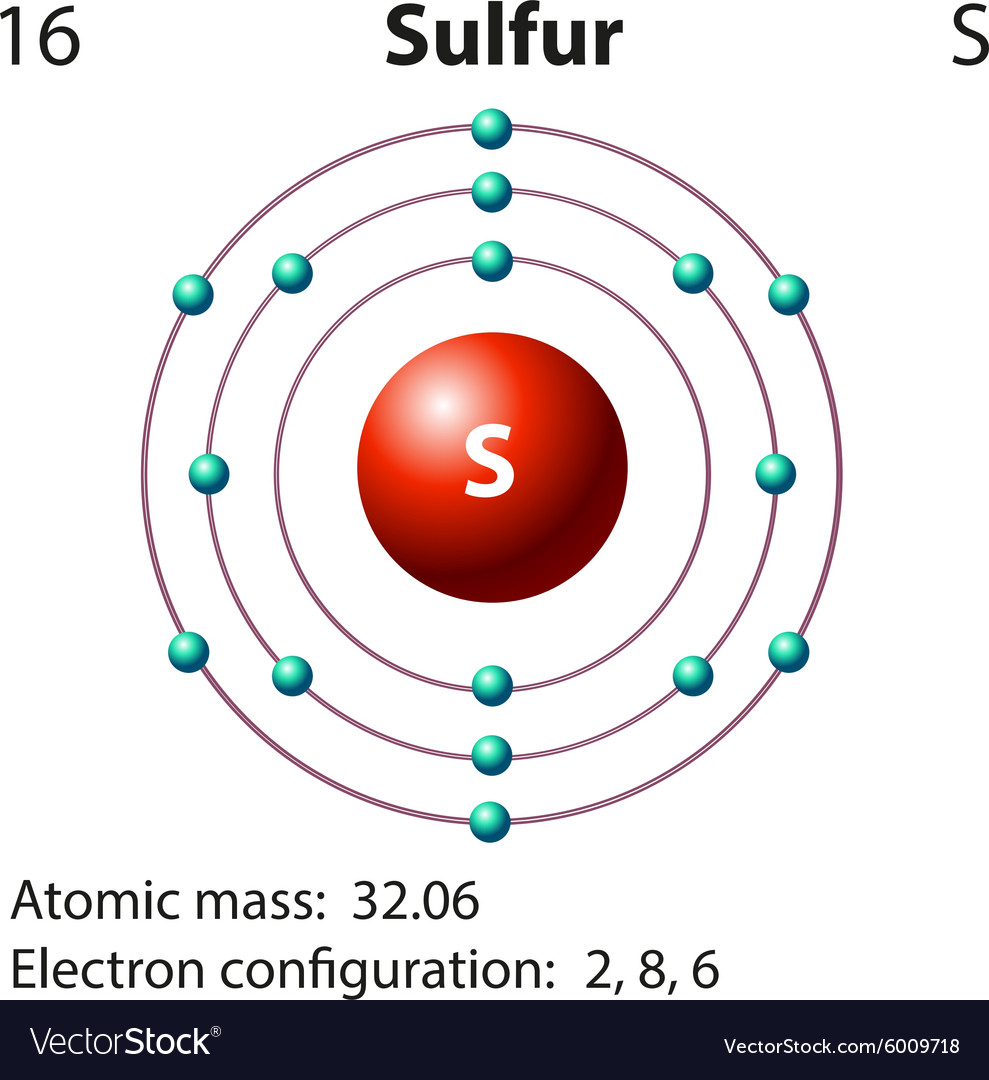
Diagram representation of the element sulfur Vector Image
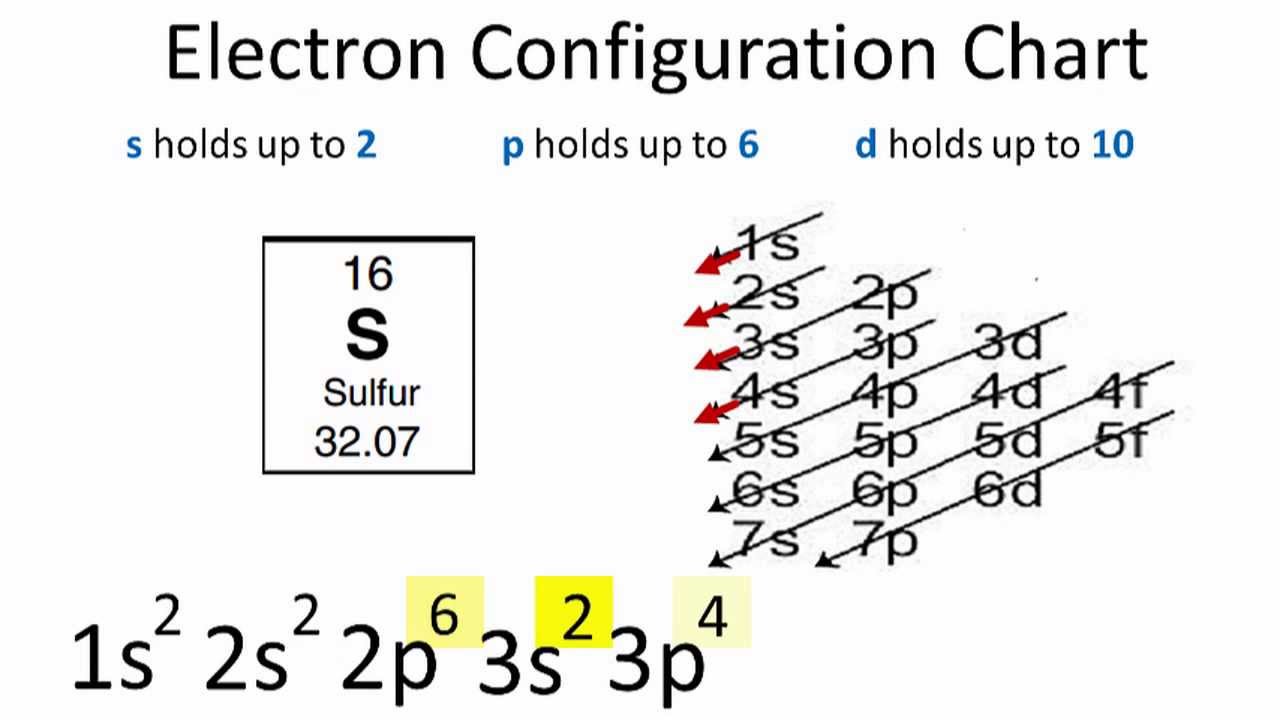
Sulfur Electron Configuration (S) with Orbital Diagram
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements

draw electron dot structure of the molecule of sulphur which is made up
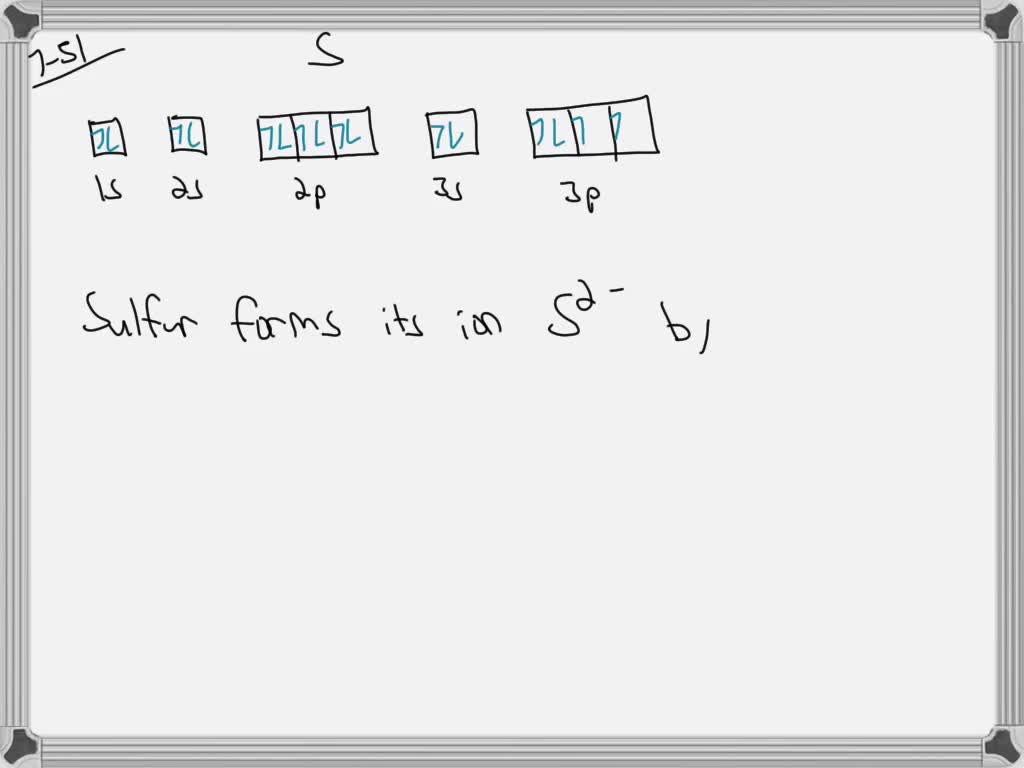
SOLVEDThe orbital notation of sulfur is shown in Figure 7.15. Explain

Sulfur S (Element 16) of Periodic Table Elements FlashCards

Draw the electrondot formula for the element sulphur YouTube

Sulfur Definition, Facts, Symbol, Allotropes, Properties, Uses
Lots Of Things Dissolve Easily In Water.
The Next Electron Is Drawn Underneath The Symbol, And Then On The Left Side.
Figure 7.9 Shows The Lewis Symbols For The Elements Of The Third Period Of The Periodic Table.
The Element Sulfur (S) Has Six Valence Electrons In Its Outer Shell.
Related Post: