Draw The Lewis Dot Structure For P
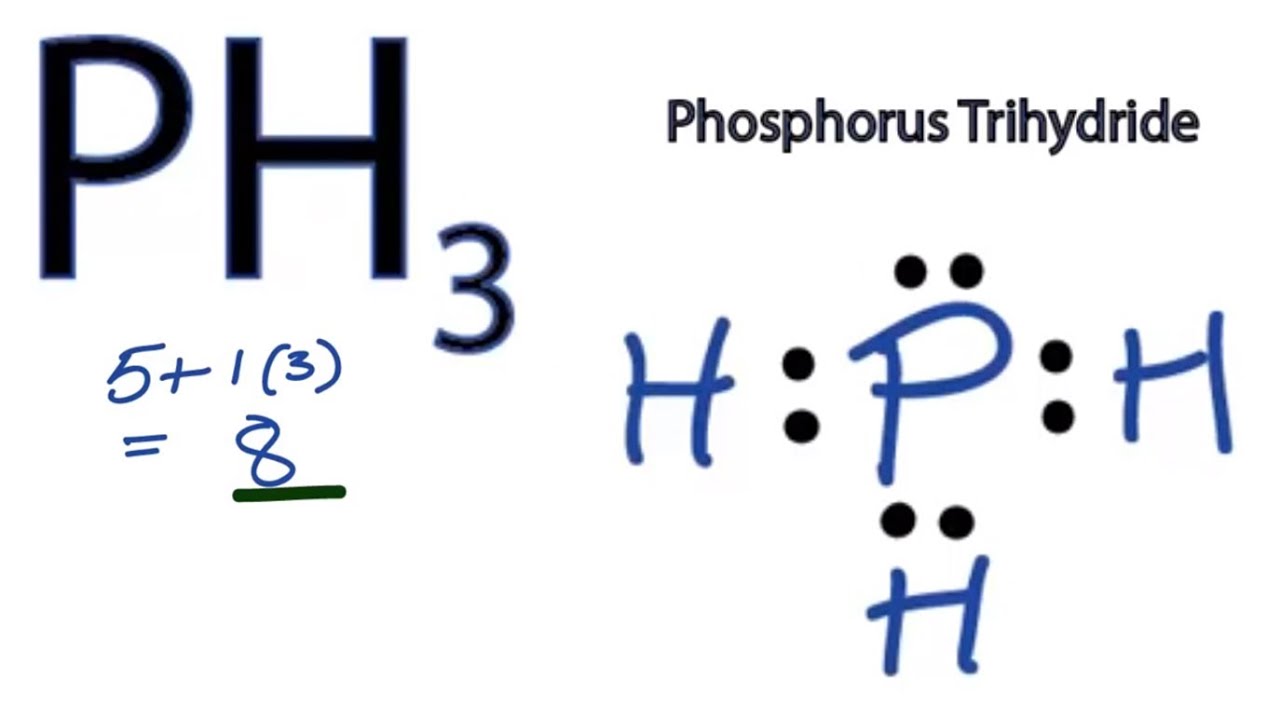
Draw The Lewis Dot Structure For P - Shared pairs of electrons are drawn as lines between atoms, while. Web in the second energy level you have s and p orbitals, and in the third energy level you have s, p, and d orbitals. Since hydrogen is in group i it has one (1) valence electron in its shell. Do not add any more atoms. Be sure to have the correct number of electrons. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. For the p (ch3) structure use the periodic table to find the total number of valence electrons for the p (ch3) molecule. The arrangement of atoms in several biologically important molecules is given here. Web 0:00 / 1:55 how to draw the lewis dot diagram of p (phosphorus) chemistnate 259k subscribers subscribe subscribed 33k views 4 years ago lewis. Web part b draw the lewis dot structure for p. Shared pairs of electrons are drawn as lines between atoms, while. Web remember that lewis dot structures are drawn for covalent (molecular) compounds that share electrons. Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. \ [\mathbf {\cdot \dot {c}}\mathbf {:}\nonumber \] with n, which has three p electrons, we put a. Draw the lewis dot structure for the hydrogen atom. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Web method 1 drawing diatomic covalent structures 1 write the atomic symbol for each atom. Stages to articulate the electron dot formula are stated beneath. Web drawing lewis dot structures and resonance. So you can fit more than eight electrons. If the species is an ion, add or subtract electrons corresponding to the charge. Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. Shared pairs of electrons are drawn as lines between atoms, while. Figure 7.9 lewis symbols illustrating the number of valence electrons. Web a lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. I show you where phosphorous is on the periodic table and how to determine how many valence electrons. As such,. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Draw the lewis dot structure for the hydrogen atom. Do not add any more atoms. Remember that lewis dot structures only give reasonable results for covalent compounds and. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Web 0:00 / 1:55 how to draw the lewis dot diagram of p (phosphorus) chemistnate 259k subscribers subscribe subscribed 33k views 4 years ago lewis. Complete the lewis structures of these molecules by adding multiple bonds and lone pairs. Draw the atoms on paper. Web remember that lewis dot structures are drawn for covalent (molecular) compounds that share electrons. I show you where phosphorous is on the periodic table and how to determine how many valence electrons. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. \ [\mathbf {\cdot \dot {c}}\mathbf {:}\nonumber \] with n,. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. Web in the second energy level you have s and p orbitals, and in the third energy level you have s, p, and d orbitals. Complete the lewis structures of these molecules by adding multiple bonds and lone pairs.. Web method 1 drawing diatomic covalent structures 1 write the atomic symbol for each atom. Figure 7.9 lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Add up the total number of valence electrons found in the entire compound. Web a lewis electron dot symbol (or electron dot diagram or. As such, the electron dot diagram for carbon is as follows: For the p (ch3) structure use the periodic table to find the total number of valence electrons for the p (ch3) molecule. Write the 2 atomic symbols side by side. Complete the lewis structures of these molecules by adding multiple bonds and lone pairs. Shows the lewis symbols for. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. Figure 7.9 shows the lewis symbols for the elements of the third period of the periodic table. We use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web science chemistry chemistry questions and answers draw the lewis dot structure for p. Complete the lewis structures of these molecules by adding multiple bonds and lone pairs. Write the 2 atomic symbols side by side. Shared pairs of electrons are drawn as lines between atoms, while. Shows the lewis symbols for the elements of the third period of the periodic table. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. The number of dots equals. Since hydrogen is in group i it has one (1) valence electron in its shell. Be sure to leave enough space between the atoms to draw your electrons and bonds. Web part b draw the lewis dot structure for p. So you can fit more than eight electrons. Remember that lewis dot structures only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, Web method 1 drawing diatomic covalent structures 1 write the atomic symbol for each atom.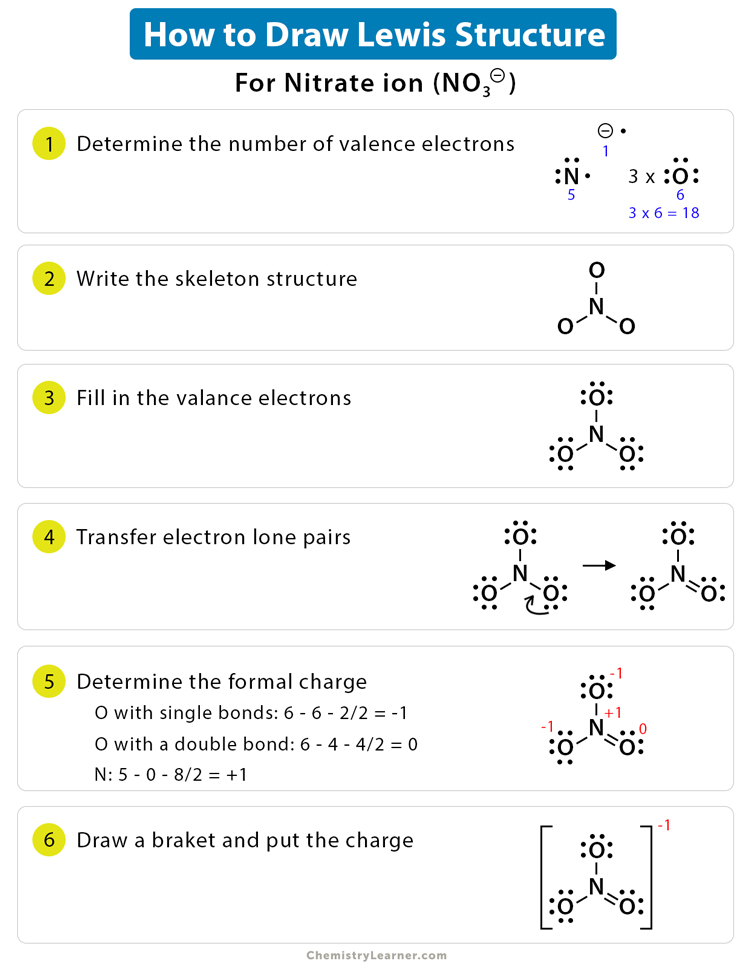
Lewis Dot Structure Definition, Examples, and Drawing

How to Draw the Lewis Dot Structure for P 3 (Phosphide ion) YouTube
Lewis Dot Diagram Phosphorus

How to Draw a Lewis Structure

Comment dessiner une représentation de Lewis Wiki Chimie
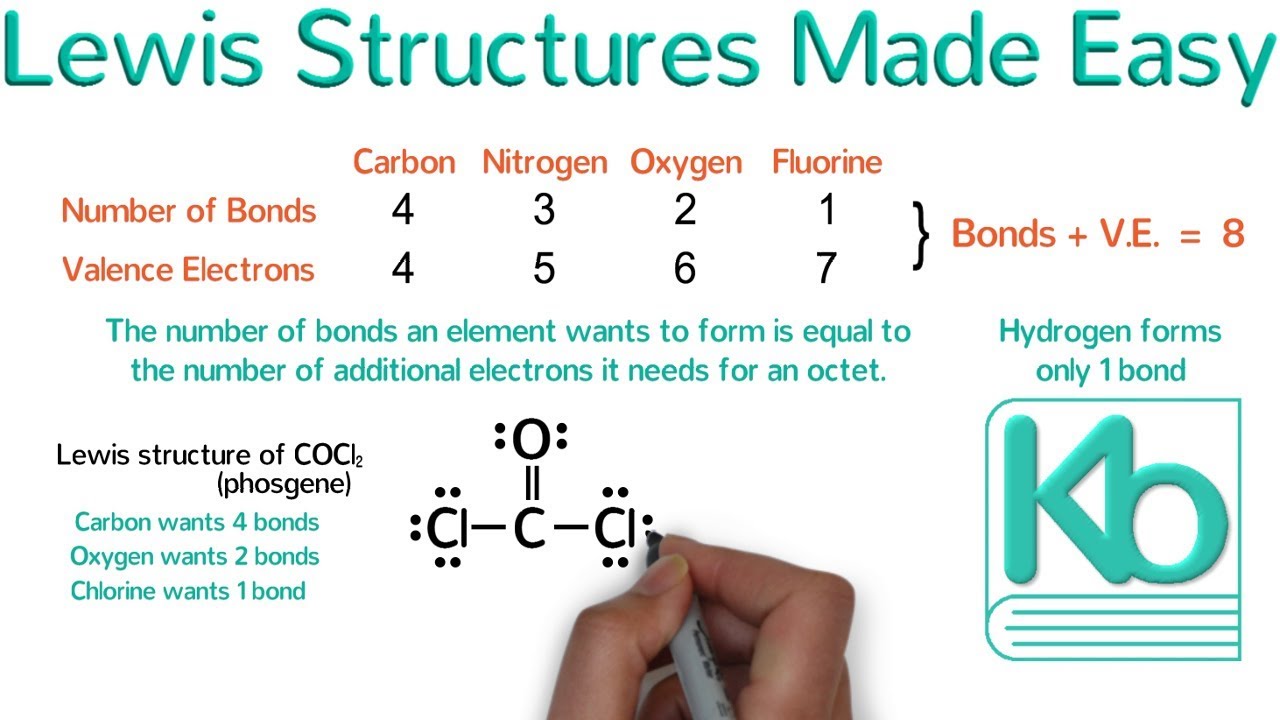
Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot

How to Draw the Lewis Dot Structure for P(CH3) Trimethylphosphine YouTube
Lewis Electron Dot Structure Calculator

3 Ways to Draw Lewis Dot Structures wikiHow
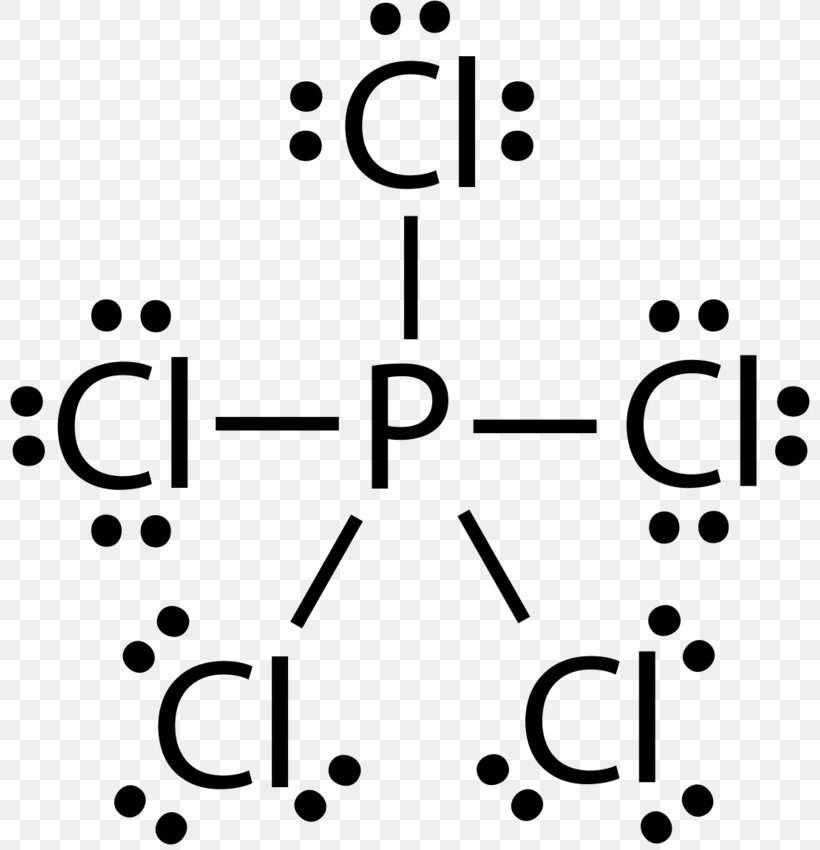
39 electron dot diagram for phosphorus
Web Drawing Lewis Dot Structures And Resonance Structures.
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
Web In The Second Energy Level You Have S And P Orbitals, And In The Third Energy Level You Have S, P, And D Orbitals.
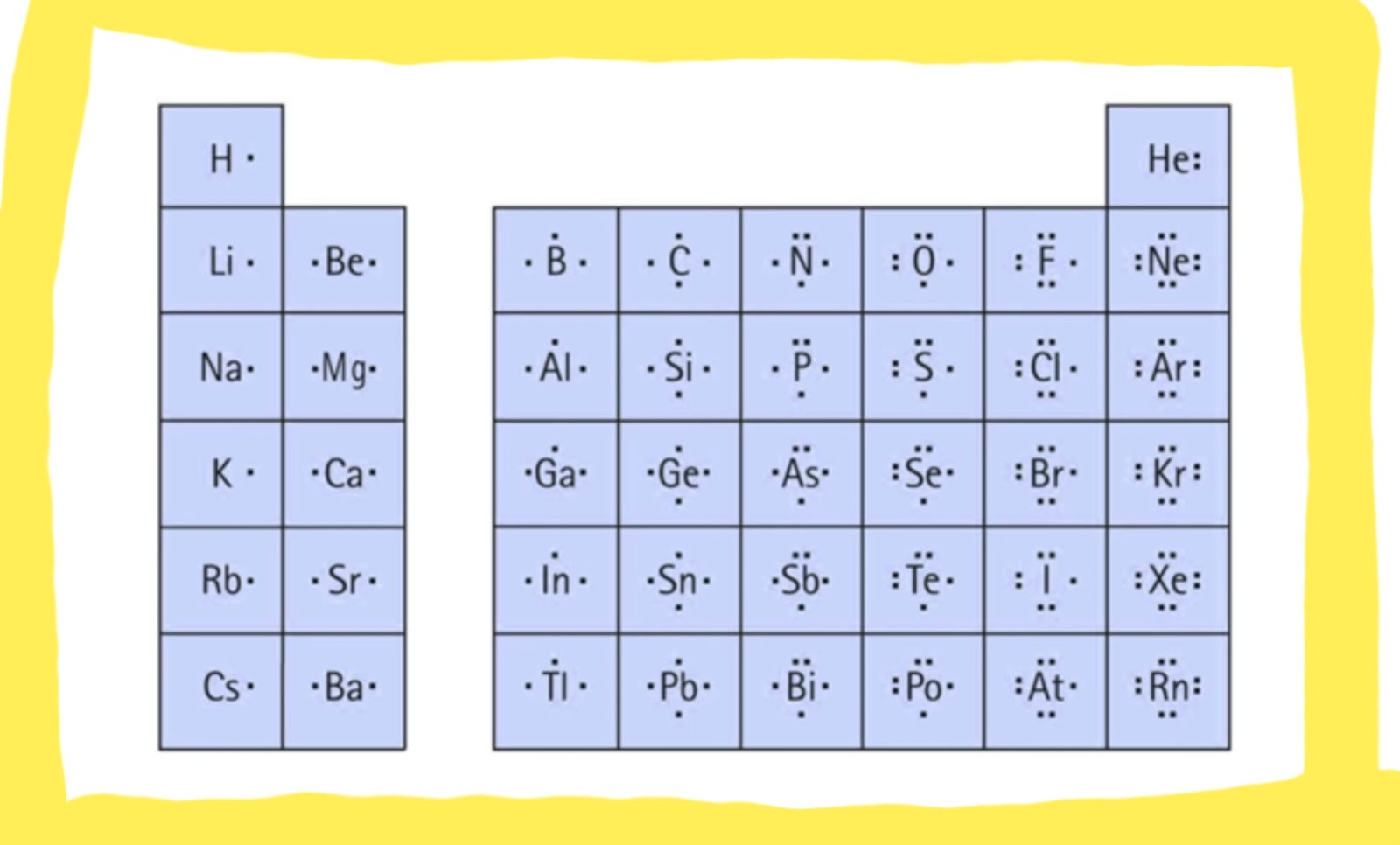
Figure 7.9 Lewis Symbols Illustrating The Number Of Valence Electrons For Each Element In The Third Period Of The Periodic Table.
Related Post: