Draw The Lewis Structure For A Oxygen O2 Molecule
Draw The Lewis Structure For A Oxygen O2 Molecule - Total number of valence electrons: I also go over shape and bond angle. The first step is to sketch the lewis structure of the o2 molecule, to add valence electrons around the two oxygen atoms, and the final step is to combine the two oxygen diatomic atoms to get the o2 lewis structure. The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Web o 2 lewis structure. How to draw the lewis structure for oxygen gas (diatomic oxygen) watch on. Web draw a skeleton structure of the molecule. Web lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Steps for writing lewis structures. Web lewis structure of o2. Web using lewis structures, we can represent this as follows: There are many things to learn when we draw the o 2 lewis structure. The o 2 molecule is diatomic, meaning that two atoms of the same element are connected in. Web lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Web we draw lewis structures to predict: Web to draw the lewis structure of o2, we first need to determine the number of valence electrons for each oxygen atom. Oxygen gas watch on steps of drawing o2 lewis structure step 1: Calculate the total number of valence electrons. In the lewis structure of o 2 molecule, a double bond is located between oxygen atoms and each oxygen. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons. Find the total valence electrons in o2 molecule in order to find the total valence electrons in o2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom. The first step is to sketch the lewis structure of. Be sure to draw all bonds and lone pairs. The o 2 lewis structure has a double bond between two oxygen atoms. Two fluorine atoms can form a molecule of f 2 in the same fashion. Drawing the lewis structure for o 2 ( dioxygen or oxygen gas) The o 2 molecule is diatomic, meaning that two atoms of the. Web in the lewis structure of o2 structure there are a total of 12 valence electrons. Web draw a skeleton structure of the molecule. I also go over shape and bond angle. Drawing the lewis structure for o 2 ( dioxygen or oxygen gas) Here, the given molecule is o2 (oxygen). Web to draw the lewis structure of o2, we first need to determine the number of valence electrons for each oxygen atom. Two fluorine atoms can form a molecule of f 2 in the same fashion. Steps for writing lewis structures. Calculate the total number of valence electrons. Be sure to draw all bonds and lone pairs. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. In the lewis structure of o 2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. Here, the given molecule is o2 (oxygen). Oxygen is in group 16 of the periodic table, so. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons. O2 is also called oxygen gas (diatomic oxygen). We place three lone pairs of electrons around each f atom, accounting for 36 electrons. Two fluorine atoms can form a molecule of f 2 in the same fashion. 1st attempt part 1 (1 point) draw the. The o 2 lewis structure has a double bond between two oxygen atoms. In the lewis structure of o 2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. Web in the lewis structure of o2 structure there are a total of 12 valence electrons. Determine the total. Note that each atom must contribute one electron to the bond. Atoms can form more than one bond. Web oxygen is a diatomic molecule and contains only two oxygen atoms. Web 6 steps to draw the lewis structure of o2 step #1: Web using lewis structures, we can represent this as follows: Here, the given molecule is o2 (oxygen). Web in the lewis structure of o2 structure there are a total of 12 valence electrons. O 2 ( oxygen) has two oxygen atoms. Web to draw the lewis structure of o2, we first need to determine the number of valence electrons for each oxygen atom. In order to make sure the outer shell of the oxygen. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. Steps for writing lewis structures. Total number of valence electrons: There are many things to learn when we draw the o 2 lewis structure. Two electrons remain, and this lone pair is placed on the xe atom: Web o 2 lewis structure. Oxygen gas watch on steps of drawing o2 lewis structure step 1: 1st attempt part 1 (1 point) draw the lewis structure for o2. Web how to draw the lewis dot structure for o2: The lewis diagram of o2 shows two oxygen atoms having twelve dots, of valence electrons. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons.
How to Draw the Lewis Dot Structure for O2 Oxygen gas YouTube
How to Draw O2 Lewis Structure? 3

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist

O2 2 Lewis Structure How to Draw the Lewis Structure for O2 2 YouTube
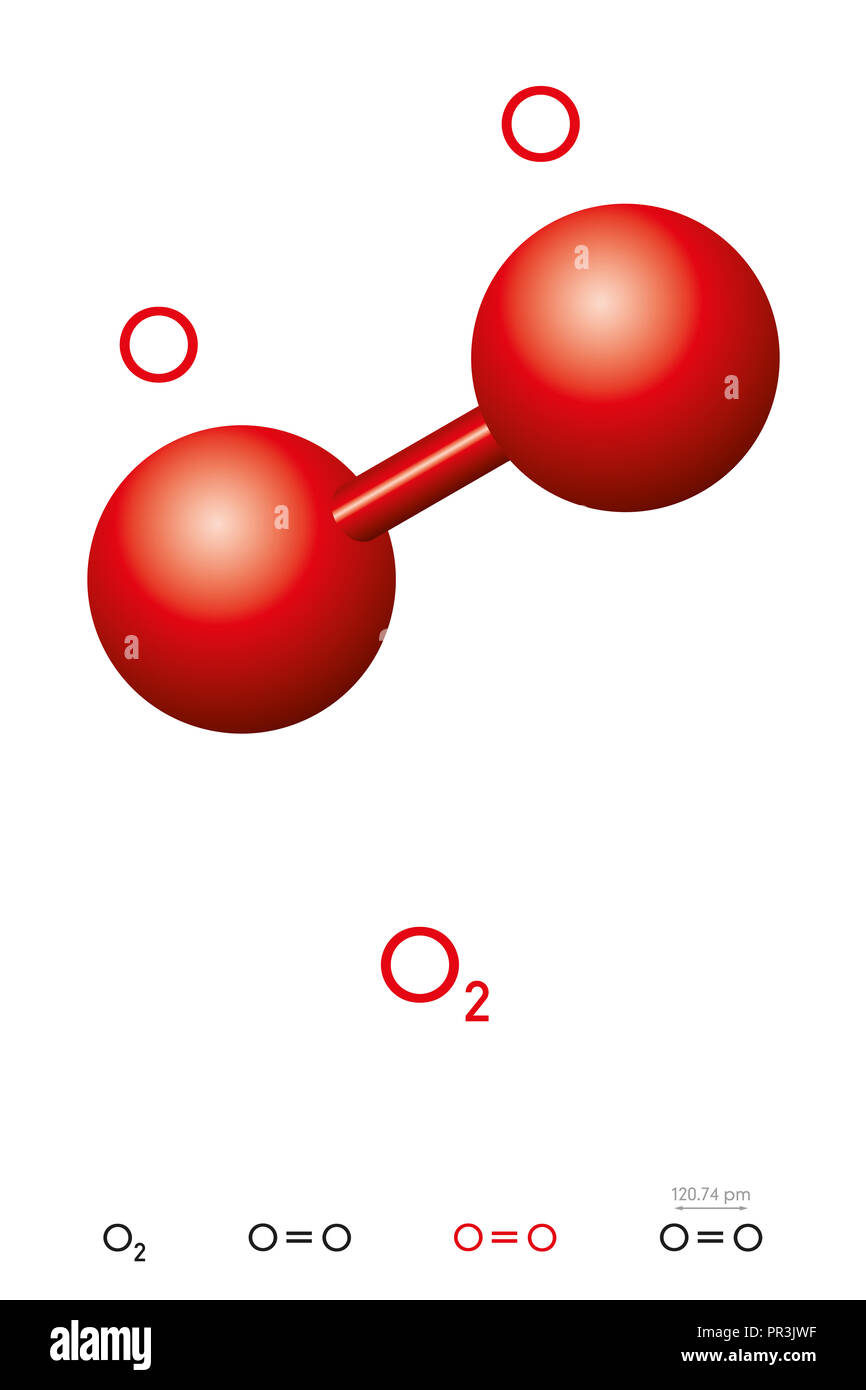
Oxygen, O2, molecule model and chemical formula. Also dioxygen
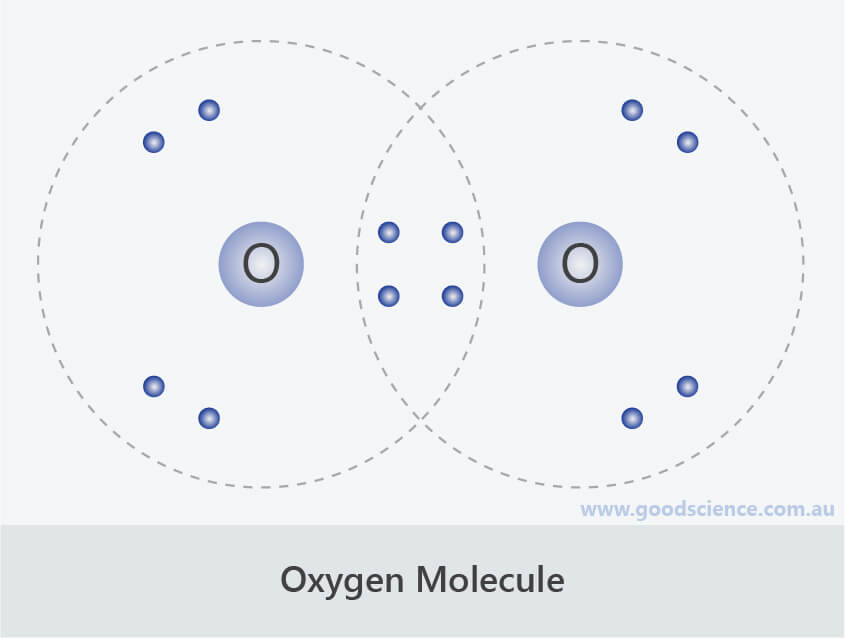
Ionic and Covalent Compounds Good Science
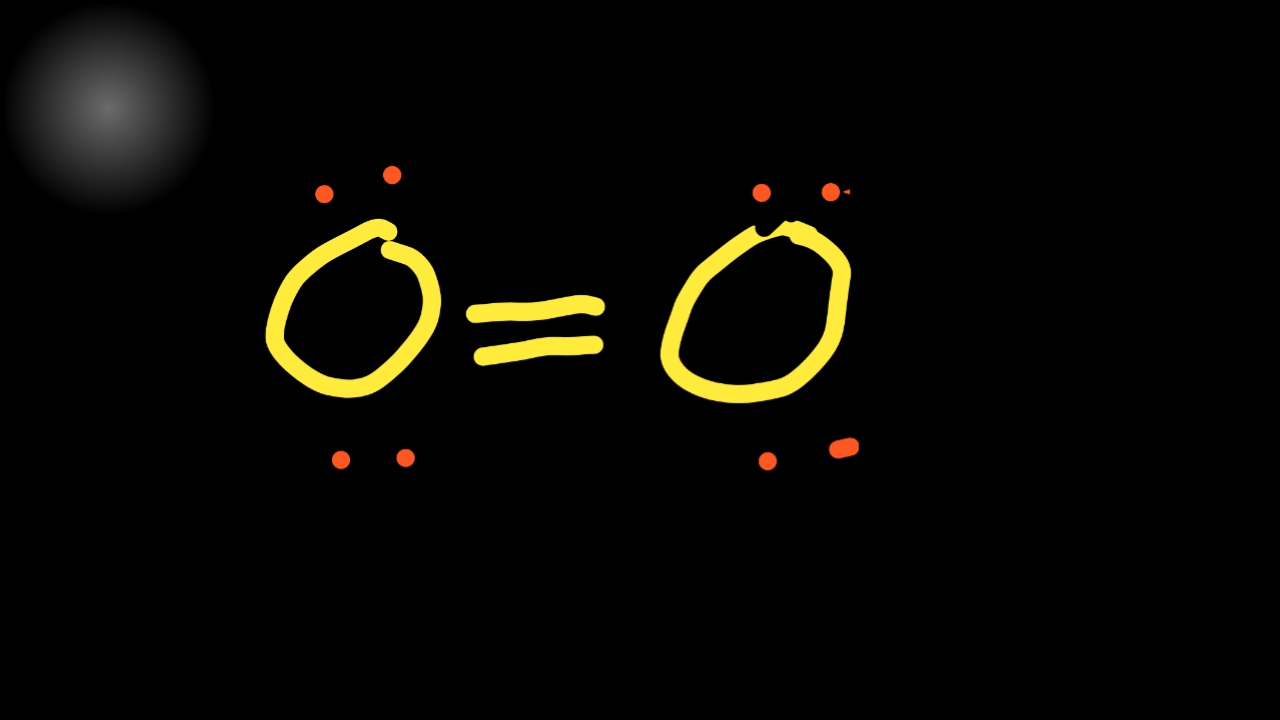
【2 Step】O2 Lewis StructureLewis Dot Structure for Oxygen(O,O2)Lewis
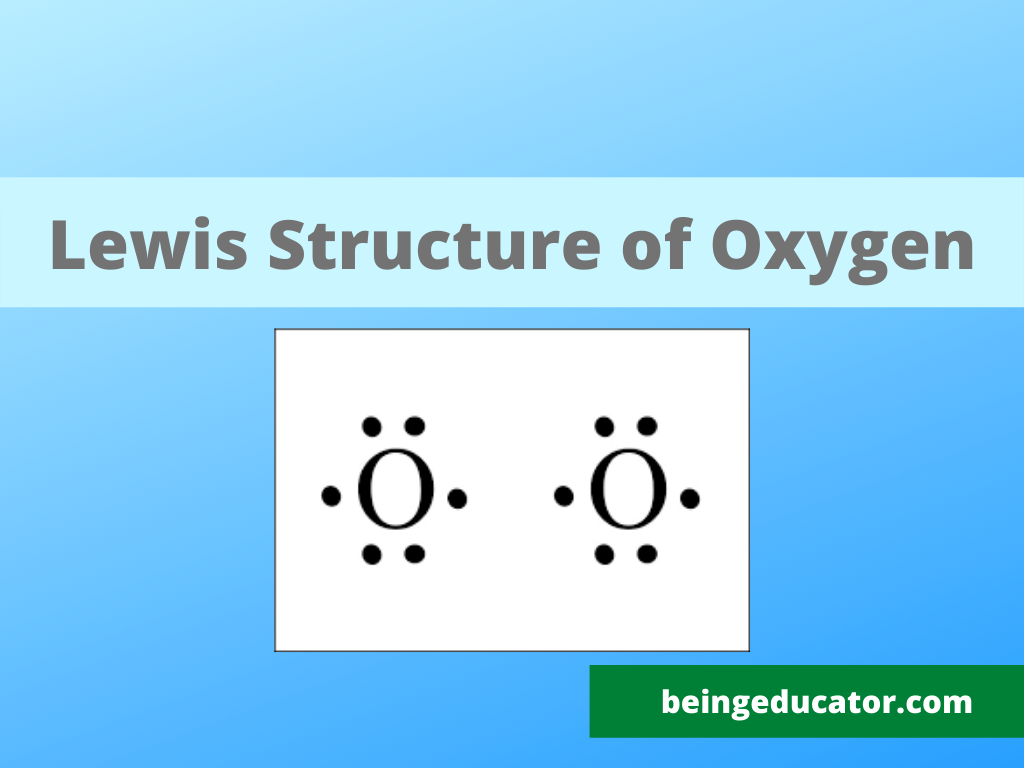
O2 Lewis Structure

O2 oxygen molecule Royalty Free Vector Image VectorStock
Here We Will Take Co 2 Molecule As An Example To Explain The Procedure Step By Step:.
Each H Atom (Group 1) Has 1 Valence Electron, And The O Atom (Group 16) Has 6 Valence Electrons, For A Total Of 8 Valence Electrons.
In This Case, There Is No Central Atom, So We Distribute The Electrons Around Both Atoms.
We Place Three Lone Pairs Of Electrons Around Each F Atom, Accounting For 36 Electrons.
Related Post: