Draw The Lewis Structure For A Sulfur Monoxide So Molecule
Draw The Lewis Structure For A Sulfur Monoxide So Molecule - Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web write lewis symbols for neutral atoms and ions. Web lewis structures, also known as lewis dot structures or electron dot structures, are diagrams that represent the valence electrons of atoms within a molecule. Draw lewis structures depicting the bonding in simple molecules. For anions, add one electron for each negative charge. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. This problem has been solved! Draw lewis structures depicting the bonding in simple molecules. Web drawing lewis structures for molecules with one central atom: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw the lewis structure for a sulfur monoxide molecule. 05:31 challenge draw the lewis structure for the molecule formed when six fluorine atoms and one sulfur atom bond covalently. Since valence electrons are typically represented as dots, these structural formulas sometimes are called. The resultant molecule is a radical because of the unpaired electron. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. For a molecule, we add the number of valence electrons on each atom in the molecule: This problem has been solved! Be sure to include all resonance structures that satisfy the octet rule. For anions, add one electron for each negative charge. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web lewis structures are structural formulas for. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. For the s2o structure use the periodic table to find the total number of valence. To draw the lewis structure for the sulfur monoxide (so) molecule, start by drawing sulfur and oxygen bonded together. The resultant molecule is a radical because of the unpaired. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web write lewis symbols for neutral atoms and ions. Web write lewis symbols for neutral atoms and ions. You'll get a detailed solution from a. Calculate the total number of valence electrons. Web lewis structures, also known as lewis dot structures or electron dot structures, are diagrams that represent the valence electrons of atoms within a molecule. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. Here, the given molecule is so (sulfur monoxide). The following procedure will. For the s2o structure use the periodic table to find the total number of valence. Here, the given molecule is so (sulfur monoxide). Web write lewis symbols for neutral atoms and ions. Calculate the total number of valence electrons. Web 6 steps to draw the lewis structure of so step #1: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw lewis structures for molecules. For a molecule, we add the number of valence electrons on each atom in the molecule: Find more chemistry widgets in wolfram|alpha. Web lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. For anions, add one electron for each negative charge. For the s2o structure use the periodic table to find the total number of valence. For a molecule, we add the number of valence electrons on each atom in the molecule: (10.4.1) + sih 4 (10.4.2) + si: In all cases, these bonds involve the sharing or transfer of valence shell. Draw the lewis structure for the sulfur trioxide (so3) molecule. Web 11k views 2 years ago. Web best matched videos solved by our expert educators. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The resultant molecule is a radical because of the unpaired electron. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web c 2 h 4 so → c 2 h 4 + so the so molecule is thermodynamically unstable, converting initially to s 2 o 2. Web write lewis symbols for neutral atoms and ions. Web this widget gets the lewis structure of chemical compounds. The resultant molecule is a radical because of the unpaired electron. Draw the lewis structure for the sulfur trioxide (so3) molecule. For a molecule, we add the number of valence electrons on each atom in the molecule: Understand the proper use of the octet rule to predict bonding in simple molecules. Draw lewis structures depicting the bonding in simple molecules. Web lewis structures, also known as lewis dot structures or electron dot structures, are diagrams that represent the valence electrons of atoms within a molecule. Sulfuric acid forms with one sulfur, four oxygen and two hydrogen. This structure displays a double bond between the sulfur and oxygen atoms, and each atom has two lone pairs. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web the so lewis structure represents the arrangement of a molecule consisting of one sulfur atom and one oxygen atom, also known as sulfur monoxide. Web drawing lewis structures for molecules with one central atom: Web best matched videos solved by our expert educators.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

How to Write the Formula for Sulfur monoxide YouTube

Draw the Lewis Structure for a Sulfur Monoxide So Molecule

Sulfur Monoxide, so, Molecule Model and Chemical Formula Stock Vector

SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
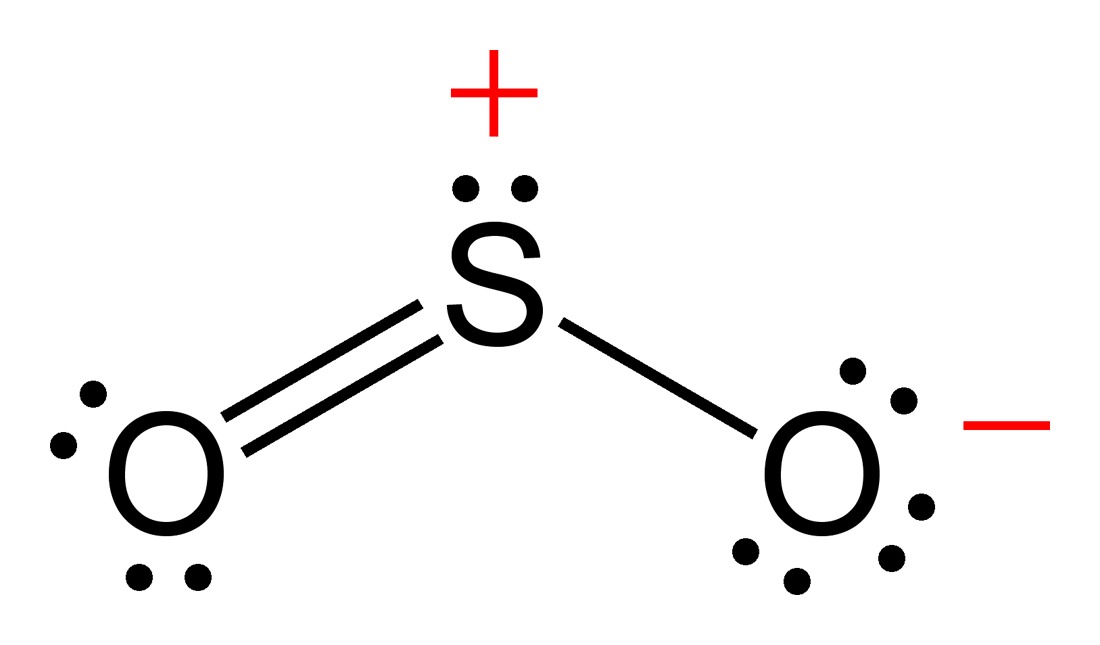
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

So sulfur monoxide molecule Royalty Free Vector Image

SOLVED Draw the Lewis structure for sulfur monoxide (SO) molecule

Sulfur monoxide YouTube
Calculate The Total Number Of Valence Electrons.
05:31 Challenge Draw The Lewis Structure For The Molecule Formed When Six Fluorine Atoms And One Sulfur Atom Bond Covalently.
Web 11K Views 2 Years Ago.
Web Writing Lewis Structures.
Related Post: