Draw The Lewis Structure For Asf3
Draw The Lewis Structure For Asf3 - Draw the lewis structure for asf3. Asf3 has a trigonal pyramidal molecular geometry and a tetrahedral electronic shape with bond angles of approximately 96°. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Find more chemistry widgets in wolfram|alpha. Calculate the total number of valence electrons. Using formal charges to determine how many bonds to make, a different perspective. Web how to draw asf 3 lewis structure? Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The following procedure can be used to draw lewis structure for simple molecules. Find the total valence electrons in asf3 molecule in order to find the total valence electrons in an asf3 molecule, first of all you should know the valence electrons. 2.draw the lewis structure for ch3+. This widget gets the lewis structure of chemical compounds. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). This problem has been solved! In order to draw the lewis. The arsenic atom goes in the center of the lewis structure since it is the least. Find more chemistry widgets in wolfram|alpha. Draw the lewis structure for ch3+. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web drawing the lewis structure for asf 3 (arsenic trihydride) viewing notes: Web drawing lewis structures for molecules with one central atom: How to draw the lewis structure for arsenic trifluoride. Web draw the most important lewis structure for asf3 and then answer the following questions. Web the first step is to sketch the lewis structure of the asf3 molecule, to add valence electrons around the arsenic atom; Web to draw the. Web steps to draw the lewis structure of asf3. There are three lone pairs on each fluorine atom, and one lone pair on the arsenic atom. In order to draw the lewis. Arsenic atom is located as the center atom. Draw the lewis structure for clo3−. Using formal charges to determine how many bonds to make, a different perspective. How to draw the lewis structure for arsenic trifluoride. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Web use these steps to correctly draw. Find the total valence electrons in asf3 molecule in order to find the total valence electrons in an asf3 molecule, first of all you should know the valence electrons. Web steps of drawing asf3 lewis structure step 1: Using formal charges to determine how many bonds to make, a different perspective. Web steps to draw the lewis structure of asf3.. Draw the lewis structure for clo3−. Draw the lewis structure for ch3+. Drawing lewis structures for bf3, pf3 and brf3; In order to draw the lewis. Arsenic atom is located as the center atom. Web use these steps to correctly draw the asf 3 lewis structure: While selecting the atom, always put the least electronegative atom at the center. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. The following procedure can be used to draw lewis structure for simple molecules. The hybridization of the central arsenic atom. Draw the lewis structure for clo3−. How to draw the lewis structure for arsenic trifluoride. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw the most important lewis structure for asf3 and then answer the following questions. Find the total valence electrons in asf3 molecule in order to find the total valence. Include all lone pairs of electrons. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Draw the lewis structure for ch3+. The second step is to add valence electrons to the three fluorine atoms, and the final step. Since they are in the same group on the periodic table they each have the same number of electrons their structures are similar. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web the lewis structure of asf3 contains three single bonds, with arsenic in the center, and three fluorines on either side. Web (a) draw lewis structures for the following chemical species: Web steps to draw the lewis structure of asf3. The arsenic atom goes in the center of the lewis structure since it is the least. _____ (b) what is the molecular geometry? Web draw the most important lewis structure for asf3 and then answer the following questions. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Find the total valence electrons in asf3 molecule in order to find the total valence electrons in an asf3 molecule, first of all you should know the valence electrons. [20%] (b) identify which of the compounds in (a) is a lewis acid, a lewis base or a radical. Asf2, asf3, asf4, asf5 and [asf6] : Draw the lewis structure for brf3. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for arsenic trifluoride (asf3). Find more chemistry widgets in wolfram|alpha. The following procedure can be used to draw lewis structure for simple molecules.
MATHS HELP
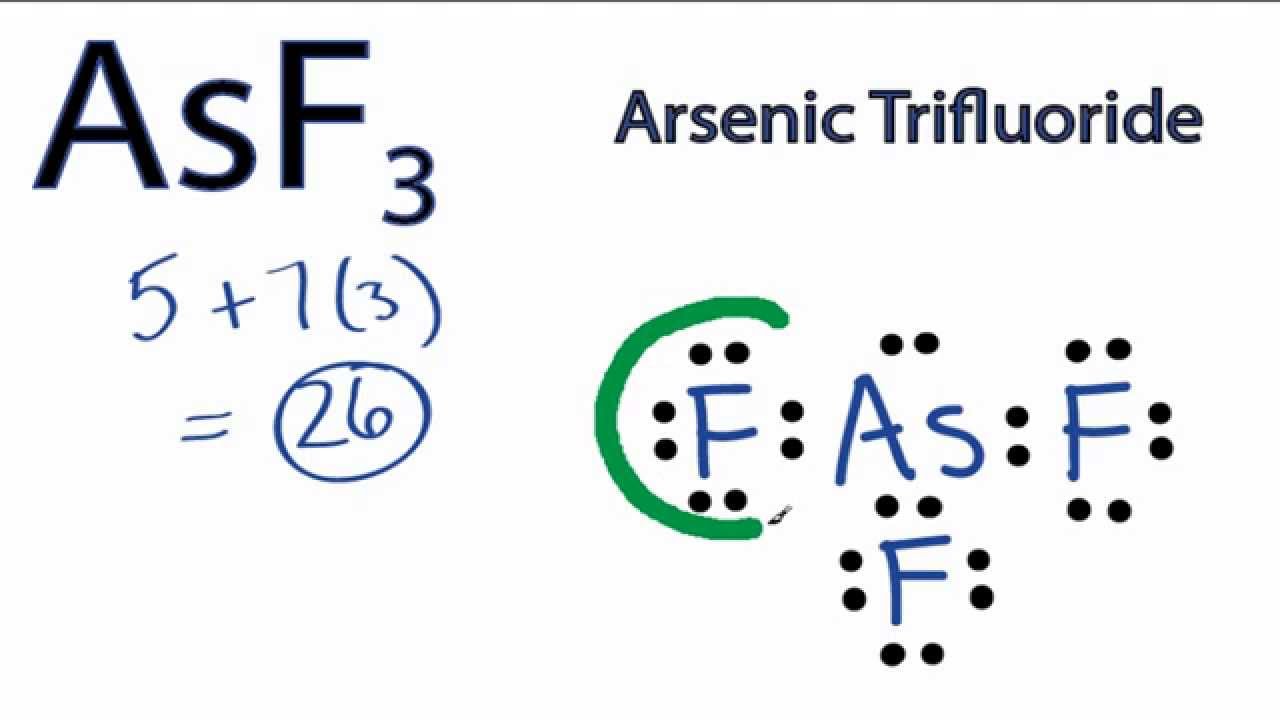
AsF3 Lewis Structure How to Draw the Lewis Structure for Arsenic
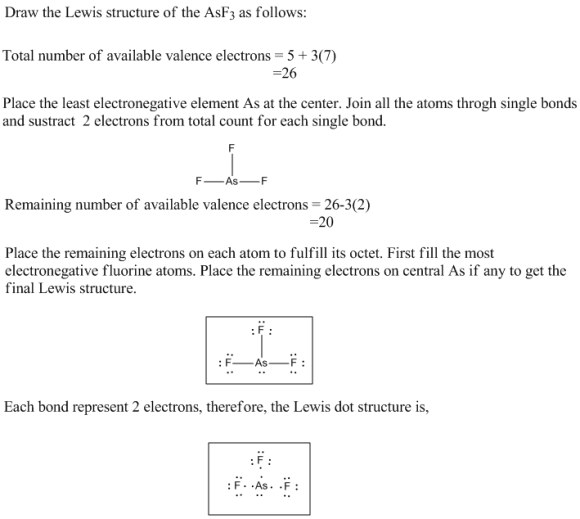
Draw the Lewis structure for AsF3 Draw the Lewis dot structure. To

AsF3 Lewis Structure (Arsenic Trifluoride) Lewis, Molecules, Electrons

AsF3 (Arsenic trifluoride) Molecular Geometry, Bond Angles YouTube

How to draw AsF3 Lewis Structure? 4
Asf3 Lewis Structure

Draw the Lewis structure for AsF3 YouTube
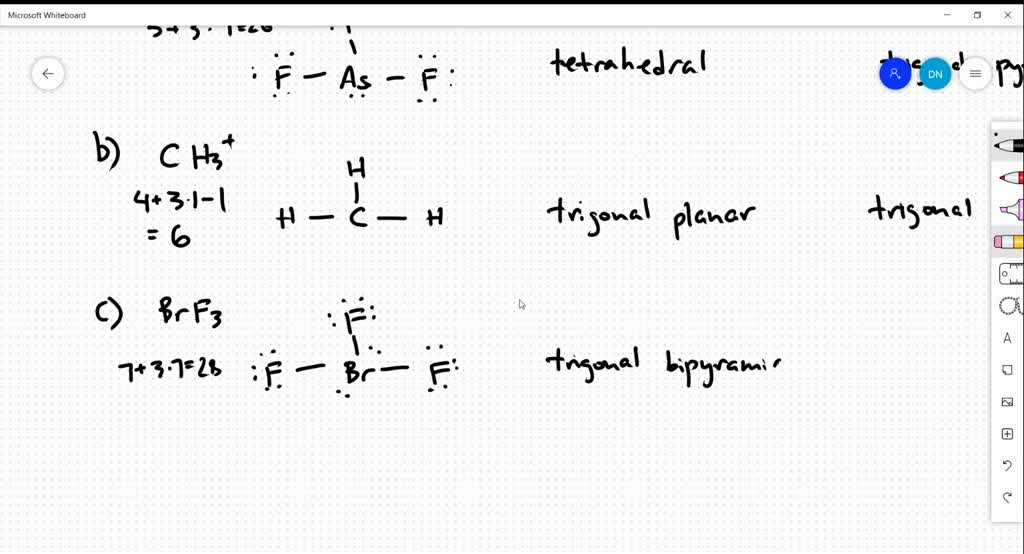
SOLVED Draw the Lewis structure for each of the following molecules or

How to draw AsF3 Lewis Structure? 3
All Other Atoms Are Bonded Directly To The Central Atom.
While Selecting The Atom, Always Put The Least Electronegative Atom At The Center.
2.Draw The Lewis Structure For Ch3+.
Lewis Structures Are Diagrams Used To Show The Valence Electrons Of Atoms And The Bonds That Form Between Them.
Related Post: