Draw The Lewis Structure For Brf3
Draw The Lewis Structure For Brf3 - Include all lone pairs of electrons. By using the following steps, you can easily draw the lewis structure of brf 3: With brf lewis structure there are a total of 14 valence electrons. Br and f are both halogens belonging to group 7 in the periodic table. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Web the lewis structure of brf3 consists of a bromine (br) atom at the center. Web drawing the lewis structure for brf. Web how to draw brf3 lewis structure? This problem has been solved! There are a total of 5 electron pairs around the central bromine atom. Drawing the lewis structure of brf3 involves understanding the concept of valence electrons, molecular geometry, chemical bonding, and the octet rule. This problem has been solved! The lewis structure for brf is similar to other structures like clf or f 2. Include all lone pairs of electrons. How many lone pairs are on the central atom of brf3? Brf3 does not follow the octet rule. Calculate the total number of valence electrons. This problem has been solved! The second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the. Web drawing the lewis structure for brf. _________polarnonpolar this problem has been solved! The lewis structure for brf is similar to other structures like clf or f 2. This problem has been solved! Web chemistry chemistry questions and answers part a draw a lewis structure for brf. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web draw the lewis electron structure of the molecule or polyatomic ion. Br and f. Web chemistry chemistry questions and answers brf3draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom. Web science chemistry chemistry questions and answers draw the lewis structure for the brf3 molecule. How to draw the lewis structure for brf (bromine monofluoride) watch on. Is brf3 polar or nonpolar? With brf lewis structure. Web chemistry chemistry questions and answers brf3draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom. Web the lewis structure of brf3 consists of a bromine (br) atom at the center. Breaking the octet rule ; Drawing the lewis structure of brf3 involves understanding the concept of valence electrons, molecular geometry, chemical. Include all lone pairs of electrons. Assign an ax m e n designation; Web here is what is needed: How many lone pairs are on the central atom of brf3? Web chemistry chemistry questions and answers part a draw a lewis structure for brf. Web chemistry chemistry questions and answers brf3draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom. Include all lone pairs of electrons. How to draw the lewis structure for brf (bromine monofluoride) watch on. Web 5 steps to draw the lewis structure of brf3 step #1: Br and f are both halogens. Drawing the lewis structure of brf3 involves understanding the concept of valence electrons, molecular geometry, chemical bonding, and the octet rule. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Web how to draw brf3 lewis structure? Drawing lewis structures for bf3, pf3 and brf3;. We draw lewis structures to predict: Part b find the direction of the. Draw the lewis dot structure for the molecule brf3. Br and f are both halogens belonging to group 7 in the periodic table. How to draw the lewis structure for brf (bromine monofluoride) watch on. Calculate the total number of valence electrons. The second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the. Web the lewis structure of brf3 consists of a bromine (br) atom at the center. The lewis structure is a visual representation of the arrangement of atoms and electrons in a molecule. Both br and f have seven valence electrons, so the lewis structure will have a total of 28 electrons, or 14 electron pairs. Br and f are both halogens belonging to group 7 in the periodic table. This problem has been solved! Draw the molecule by placing atoms on the grid and connecting them with bonds. By using the following steps, you can easily draw the lewis structure of brf 3: Assign an ax m e n designation; Is brf3 polar or nonpolar? Web chemistry chemistry questions and answers brf3draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom. We draw lewis structures to predict: Web 5 steps to draw the lewis structure of brf3 step #1: Drawing lewis structures for bf3, pf3 and brf3; Web here is what is needed:
Structure de Brf3 Lewis, caractéristiques 13 faits à connaître

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Leave a Comment Cancel Reply
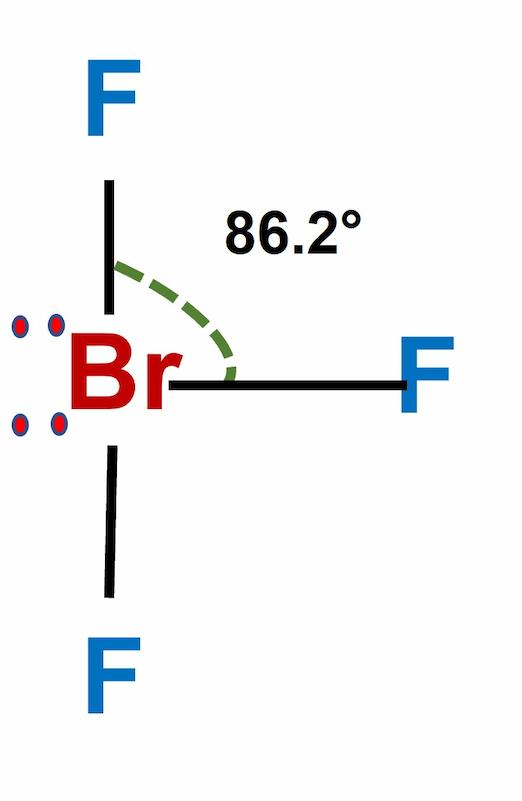
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight

Formal charge on bromine atom of BrF3 molecule = (7 4(6/2)) =0

BrF3 Molecular Geometry Science Education and Tutorials
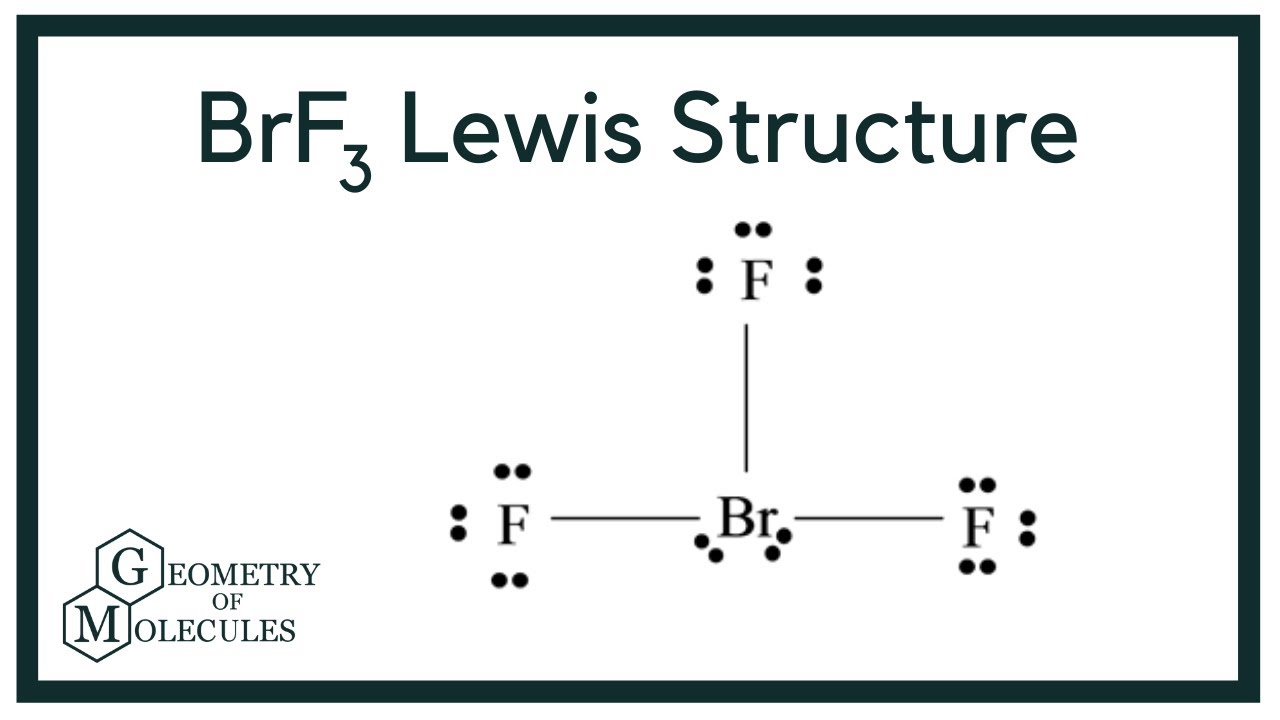
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

Estructura de Brf3 Lewis, características 13 datos que debe conocer
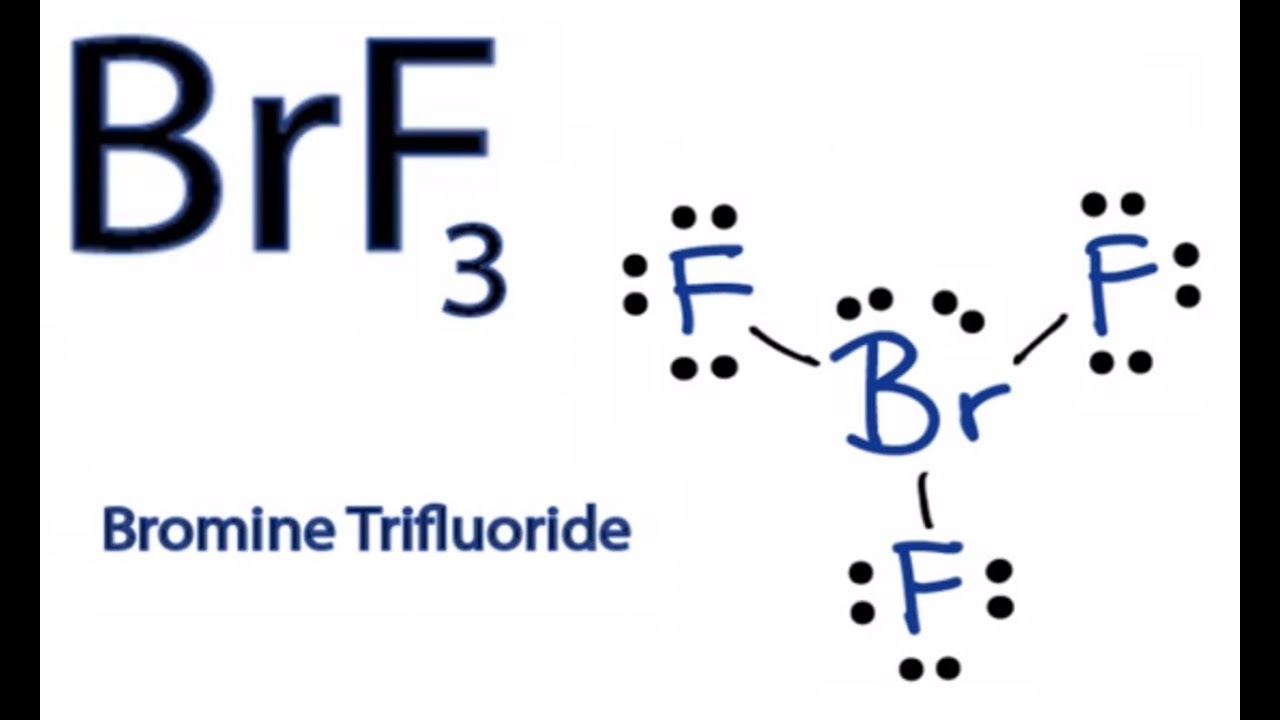
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube
Web Chemistry Chemistry Questions And Answers Part A Draw A Lewis Structure For Brf.
Web Steps To Form Brf3 Lewis Structure Step 1:.
Draw The Lewis Dot Structure For The Molecule Brf3.
#1 First Draw A Rough Sketch #2 Mark Lone Pairs On The Atoms #3 Calculate And Mark Formal Charges On The Atoms, If Required Let’s Discuss Each Step In More Detail.
Related Post: