Draw The Lewis Structure For N2
Draw The Lewis Structure For N2 - Web because n 2 is a simple molecule, it is extremely easy to draw the lewis structure of it. O + 2 (f) = of 2. Determine the central atom in n2, both nitrogen atoms are equivalent, so there is no distinct. Calculate the total number of valence electrons. Put the least electronegative atom in the center. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. Web lewis structures for covalent molecules: Web steps to properly draw the n 2 lewis structure, follow these steps: Since n is a member of the group 5a (based on the periodic table), the number of electrons in its outermost shell must be 5. Firstly, check out the atomic number of each atom from the periodic table. The lewis structure helps us visualize the arrangement of atoms and electrons in a molecule. Since n is a member of the group 5a (based on the periodic table), the number of electrons in its outermost shell must be 5. The lewis structure for n 2 looks easy at first. Determine the total number of valence electrons the first step. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom. Since there are two nitrogen atoms in n 2 you. The n 2 lewis structure indicates that the n 2 molecule is perfectly symmetric. The lewis structure helps us visualize the arrangement of atoms and electrons in a molecule. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. Since there are two nitrogen atoms in n 2 you have a total of ten valence electrons. Web lewis structures for covalent molecules: Here, the given molecule is n2 (nitrogen gas). In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. Web each nitrogen atom has five valence electrons. Web steps to properly draw the n 2 lewis. Send feedback | visit wolfram|alpha get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web 17k views 2 years ago. O + 2 (f) = of 2. Web drawing the lewis structure of n2 involves finding the total valence electrons, selecting the central atom, connecting the atoms with a bond, distributing the remaining electrons,. Web representing a covalent bond using lewis structures. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons in the molecule. Web here we draw the n2 lewis structure, molecular geometry, and hybridization of nitrogen molecule. The lewis structure for n 2 looks easy at first. Draw a skeleton structure. Find total number of electrons of the valance shells of nitrogen atoms determine total electrons pairs existing as lone pairs and bonds Web representing a covalent bond using lewis structures. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom. Web to draw the n2 lewis structure, you can follow these. Determine the central atom in n2, both nitrogen atoms are equivalent, so there is no distinct. The lewis structure for n 2 looks easy at first. Since n is a member of the group 5a (based on the periodic table), the number of electrons in its outermost shell must be 5. Web added jun 9, 2014 by webtester in chemistry. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons in the molecule. Determine the total number of valence electrons in the molecule by adding the valence electrons of each nitrogen atom. Drawing lewis structures for molecules with one central atom: For the n2 structure use the periodic table to. The first thing we need to do when drawing a lewis structure is determine the total number of valence electrons in the molecule. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. The lewis diagram for n 2 is as. Since there are two nitrogen atoms in n 2 you have a total of ten valence electrons to work with. Put the least electronegative atom in the center. While selecting the atom, you have to put the least electronegative atom at the center. The total number of electrons is 4 x 2(1) + 6 = 12 electrons. Web added jun 9, 2014 by webtester in chemistry this widget gets the lewis structure of chemical compounds. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. Web the bond between the two nitrogen atoms is a triple bond. In ch 2 o, the central atom is surrounded by two different types of atoms. Therefore, n 2 is a nonpolar substance. Calculate the total number of valence electrons. Since n is a member of the group 5a (based on the periodic table), the number of electrons in its outermost shell must be 5. Find the total valence electrons for the n2 molecule. The lewis structure helps us visualize the arrangement of atoms and electrons in a molecule. Send feedback | visit wolfram|alpha get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web this is the total number of electrons that must be used in the lewis structure. Rules to draw lewis structure.
N2 Lewis Structure How To Draw The Lewis Structure For N2

What is the Lewis structure of N2? Socratic

How to draw N2 Lewis Structure Science Education and Tutorials

Lewis Dot Structure For N2 Draw Easy

Lewis Structure for N2 How To Discuss
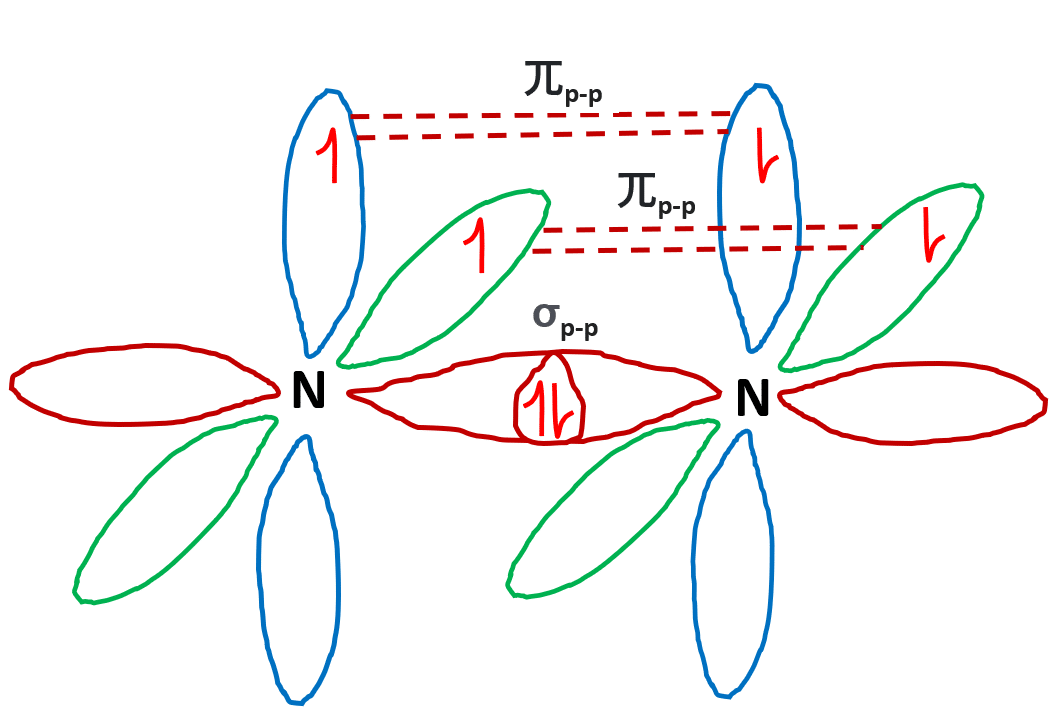
N2 Lewis Structure Hybridization & Molecular Geometry What's Insight
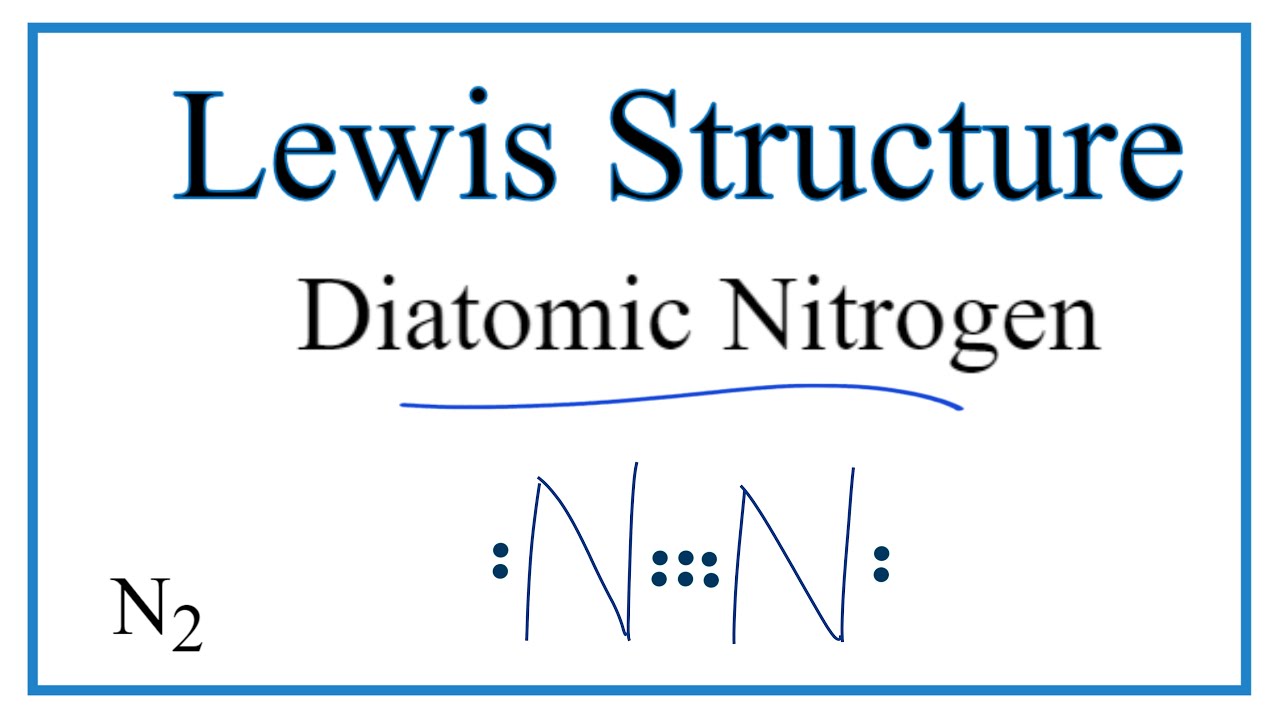
How to Draw the Lewis Dot Structure for Diatomic Nitrogen (N2) YouTube

N2 Lewis Structure How to Draw the Lewis Structure for N2 Nitrogen Gas
Lewis Electron Dot Diagram Of N2 slide share
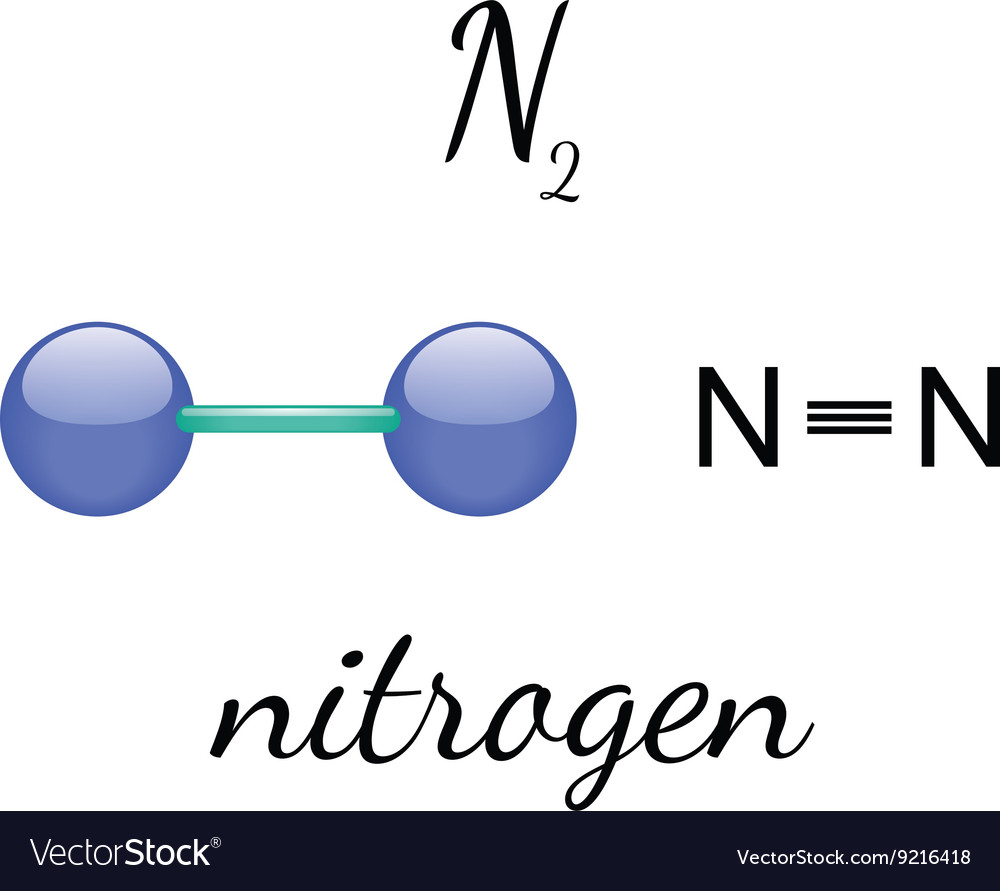
N2 nitrogen molecule Royalty Free Vector Image
Follow Along Using The Transcript.
O + 2 (F) = Of 2.
In Any Molecule Or Ion With The General Formula Abn , The Unique Atom (A) Is In The Center And All Of The B Atoms Are Attached To A.
Here Is The Electron Dot Structure For A Single N Atom:
Related Post:
