Draw The Lewis Structure For Sif4
Draw The Lewis Structure For Sif4 - Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [ (2) (1) + 4 + 6] = 12 valence electrons. Web step method to draw lewis structure of silicon trtrafluoride. Find the total valence electrons in sif4. Web the first step is to sketch the molecular geometry of the sif4 molecule, to calculate the lone pairs of the electron in the central silicon atom; Six electrons are used, and 6 are left over. Web chemistry questions and answers. For the sih4 structure use the periodic table to find the total number of valence electrons. Silicon has four valence electrons, and each fluorine has seven valence electrons. Part b draw the lewis structure for co. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) And that is 4 plus 28, is 32. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. Web the first step is to sketch the molecular geometry of the sif4 molecule, to. Part b draw the lewis structure for co. Six electrons are used, and 6 are left over. Calculate the total number of valence electrons. Draw a lewis structure for each of the following molecules or ions.\ \mathrm {hobr} hobr For the sih4 structure use the periodic table to find the total number of valence electrons. Web chemistry chemistry questions and answers 1. Web 46k explore the polar molecule in chemistry. Web step method to draw lewis structure of silicon trtrafluoride. First, draw a lewis structure for sif4. Web 6,137 solutions find the length of each missing side in a right triangle to the nearest tenth. Web chemistry chemistry questions and answers 1. While selecting the center atom, always put the least electronegative atom at the. Silicon has four valence electrons, and each fluorine has seven valence electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web to draw the sif4 lewis structure, follow these steps: Learn about its characteristics and how to determine the polarity of a molecule. 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. Web steps of drawing sif4 lewis structure step 1: You may use a calculator. Web in this article, we are going to discuss the chemical bonding of. Web chemistry chemistry questions and answers 1. Six electrons are used, and 6 are left over. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw a lewis structure for (a) sif4; The second step is to calculate the sif4 hybridization, and the third step is to give perfect notation for the sif4 molecular geometry. Web in this article,”sif4 lewis structure”, different facts like lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. (c) cof2 (c is central) show transcribed image text. Arrangement of atoms shown. You may use a calculator. Draw a lewis structure for (a) sif4; Here’s the best way to solve it. Draw a lewis structure for each of the following molecules or ions.\ \mathrm {hobr} hobr Web steps of drawing sif4 lewis structure step 1: Arrangement of atoms shown below, dashed lines show connections between atoms. The second step is to valence electron to the fluorine atom, and the final step is to combine the step1 and step2 to get the ccl4 lewis structure. Arrangement of atoms shown below, dashed lines show connections between atoms. Here, the given molecule is sif4 (silicon tetrafluoride). Web by. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. Web chemistry chemistry questions and answers 1. The second step is to valence electron to the fluorine atom, and the final step is to combine the step1. Related to this question for sf3+,. And that is 4 plus 28, is 32. While selecting the center atom, always put the least electronegative atom at the. First, draw a lewis structure for sif4. Web by using the following steps, you can easily draw the lewis structure of sif 4: Part b draw the lewis structure for co. Web to draw the sif4 lewis structure, follow these steps: 1 point for the correct selections (assessed when you answer) and 5 points for the lewis structure on your work. The second step is to valence electron to the fluorine atom, and the final step is to combine the step1 and step2 to get the ccl4 lewis structure. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure for sif4; Web we're going to do the lewis structure for sif4. Web part a draw the lewis structure for sih4. Web the first step is to sketch the molecular geometry of the sif4 molecule, to calculate the lone pairs of the electron in the central silicon atom; Web a video explanation of how to draw the lewis dot structure for silicon tetrafluoride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and. Here’s the best way to solve it.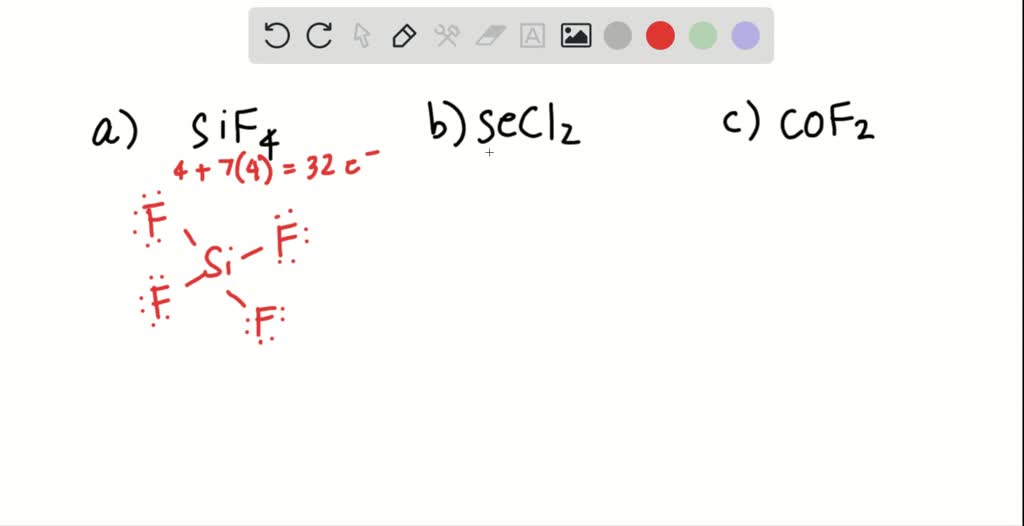
SOLVED Draw a Lewis structure for (a) SiF4; (b) SeCl2; (c) COF2 (C is
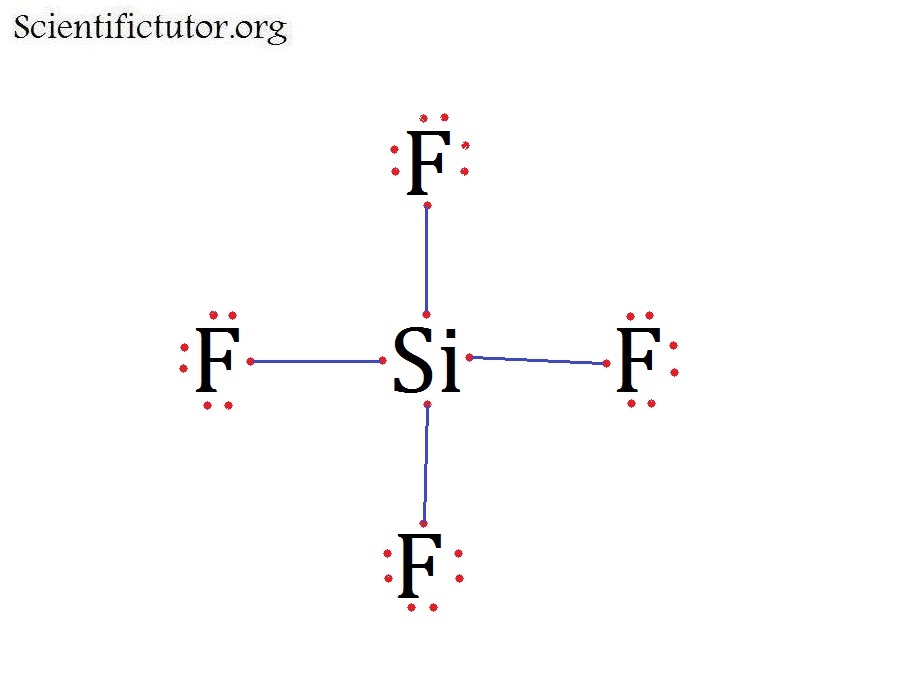
Chem Molecular Shape (Molecular Geometry) Scientific Tutor
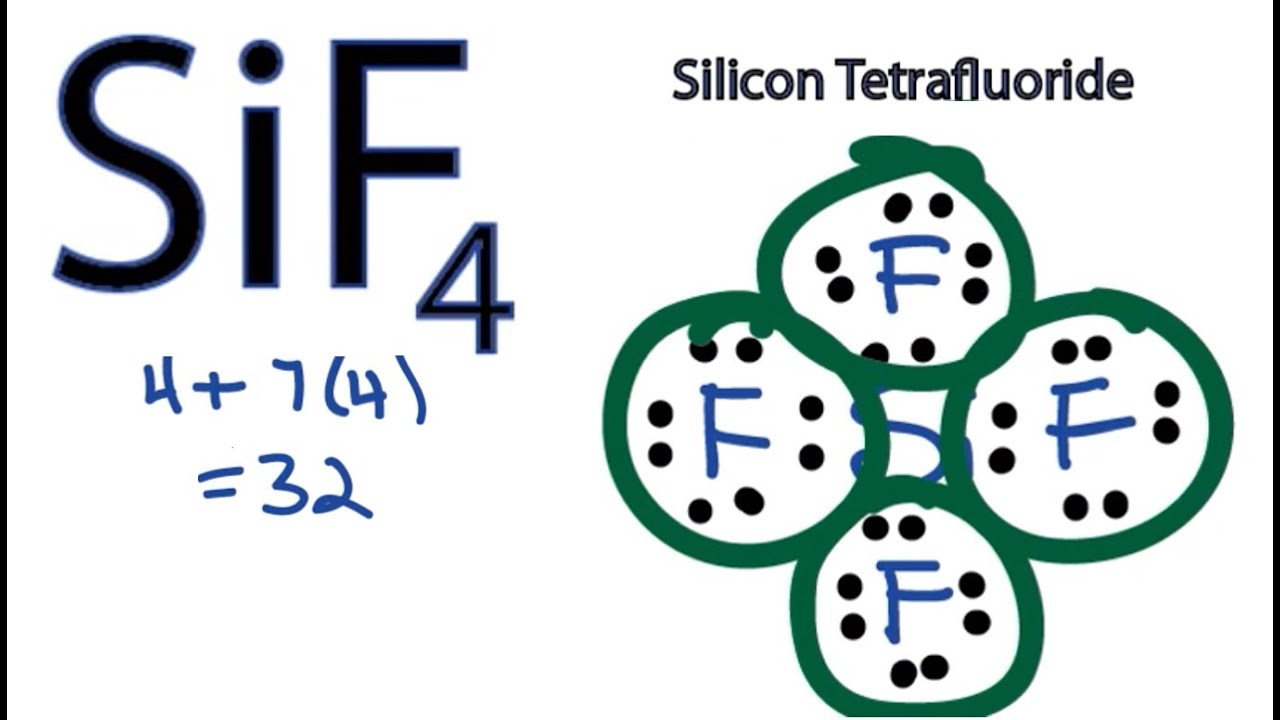
SiF4 Lewis Structure How to Draw the Dot Structure for SiF4 YouTube

Lewis Structure For Sif4
Lewis Structure For Sif4

Sif4 Lewis Structure

Sif4 Lewis Structure

How to draw SiF4 Lewis Structure? Science Education and Tutorials
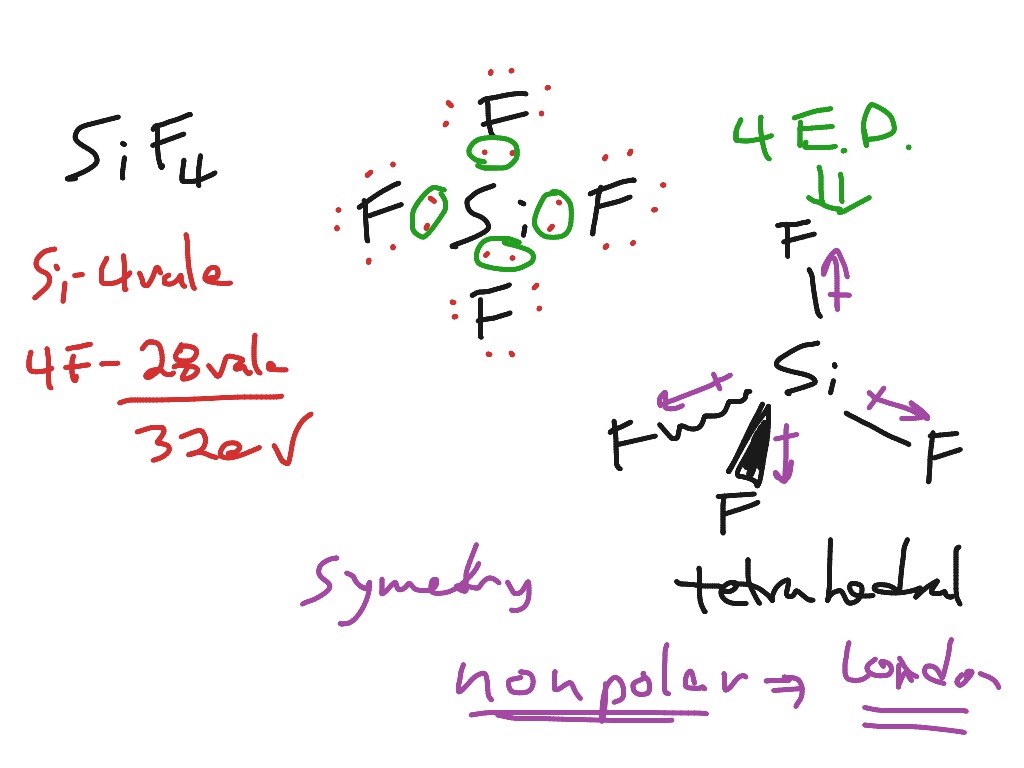
Sif4 Lewis Structure

Draw The Lewis Structure For Sif4 Drawing Easy
Six Electrons Are Used, And 6 Are Left Over.
#1 Draw Skeleton #2 Show Chemical Bond #3 Mark Lone Pairs #4 Calculate Formal Charge And Check Stability (If Octet Is Already Completed On Central Atom)
On The Periodic Table, Silicon Is In Group 4, Sometimes Called 14, So It's Got 4 Valence Electrons.
Draw A Lewis Structure For Each Of The Following Molecules Or Ions.\ \Mathrm {Hobr} Hobr
Related Post: