Draw The Lewis Structure For The Tellurium Tetrabromide Tebr4 Molecule
Draw The Lewis Structure For The Tellurium Tetrabromide Tebr4 Molecule - Not the question you’re looking for? Web tebr4 lewis structure has a tellurium atom (te) at the center which is surrounded by four bromine atoms (br). Halides structure of tellurium tetrachloride, tetrabromide and tetraiodide. What is tef 4 and what is it used for? Edit view insert format tools table v 12pt paragraph β ι ο α i previous question next question Web tebr 4 (tellurium tetrabromide) has one tellurium atom and four bromine atoms. (l625) toxin and toxin target database (t3db) 1 structures 1.1 2d structure structure search Web tellurium tetrabromide ( te br 4) is an inorganic chemical compound. For a complete tutorial on drawing lewis structures, see my video: It has a similar tetrameric structure to tecl 4. Te is also called tellurium. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Halides structure of tellurium tetrachloride, tetrabromide and tetraiodide. Web h 2 te is unstable, whereas salts of its conjugate base [teh] − are stable. For the tecl4 structure use the periodic table to find the total number of. Web molecular formula brte average mass 447.216 da monoisotopic mass 445.579529 da chemspider id 74282 more details: Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock. To begin, you must know two essential rules for drawing. Ć c q х this problem has been solved! Determine the formal charge on each atom in the. To begin, you must know two essential rules for drawing. Web the lewis structure has tellurium as the central atom, with 4 fluorine atoms attached. You need to show complete solution map in your quiz 3 honor pledge to receive full credit). Х this problem has been solved! You can predict the bond angles of tellurium tetrachloride by looking at. View the full answer step 2 unlock answer unlock previous question next question transcribed image text: Web the lewis structure of tebr4 contains four single bonds, with tellurium in the center, and four bromines on either side. For the tebr2 structure use the periodic table to find the total number of valence electrons for the tebr2. The binds are denoted. The binds are denoted by lines and electrons by dots. Web tebr 4 (tellurium tetrabromide) has one tellurium atom and four bromine atoms. Tellurium (te) has 6 valence electrons, and each bromine (br) atom has 7 valence electrons. Web tef4 (tellurium tetrafluoride) lewis structure | how to draw the lewis structure for tef4. For the tecl2 structure use the periodic. Х this problem has been solved! The tellurium atom (te) is at the center and it is surrounded by 4 bromine atoms (br). Web molecular formula brte average mass 447.216 da monoisotopic mass 445.579529 da chemspider id 74282 more details: What is tef 4 and what is it used for? Web instant answer step 1/5 1. There is 1 lone pair on the tellurium atom (te) and 3 lone pairs on all the four bromine atoms (br). Web lewis structure of tebr4 contains four single bonds between the tellurium (te) atom and each bromine (br) atom. You'll get a detailed solution from a subject matter expert. Web tef4 (tellurium tetrafluoride) lewis structure | how to draw. (a) brf5 (b) if3 (c) ibr2 (d). Web the lewis structure of tebr4 contains four single bonds, with tellurium in the center, and four bromines on either side. To begin, you must know two essential rules for drawing. The tellurium atom has 1 lone pair while all the four bromine atoms have 3 lone pairs. Draw a lewis structure for. Tellurium tetrafluoride, tef4, is a stable, white, hygroscopic crystalline solid and is one of two. Count the total number of valence electrons: Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock. Tellurium (te) has 6 valence electrons, and each bromine (br) atom has 7 valence electrons. Tellurium tetrabromide have one tellurium atom covalently bonded. Draw the lewis structure for the tellurium tetrabromide (tebr 4) molecule. Bromine is a halogen element with the symbol br and atomic number 35. You need to show complete solution map in your quiz 3 honor pledge to receive full credit). Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Draw the. Contents steps #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability related What is tef 4 and what is it used for? It has a similar tetrameric structure to tecl 4. Web tellurium tetrabromide ( te br 4) is an inorganic chemical compound. Web the lewis structure of tebr4 contains four single bonds, with tellurium in the center, and four bromines on either side. Draw a lewis structure for each of the following molecules or ions: Web expert answer transcribed image text: Web instant answer step 1/5 1. View the full answer step 2 unlock answer unlock previous question next question transcribed image text: This means that te has an expanded octet of ten, which is possible because. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. Let’s draw and understand this lewis dot structure. The tellurium atom will the the central. Diatomic bromine does not occur naturally, but bromine salts can be found in crustal rock. Web description tellurium tetrabromide is a chemical compound of tellurium and bromine. For a complete tutorial on drawing lewis structures, see my video:
Lewis Dot Diagram For Tellurium
[Solved] 3A. Draw the Lewis structure for TeBr4. What is the molecule's

TeF4(Tellurium Tetrafluoride) Lewis Structure How to Draw the Lewis

Tellurium bromide(TeBr4), (T4) Tellurium bromide(TeBr4), (T4) 10031

Lewis Dot Diagram For Tellurium
[Solved] 3A. Draw the Lewis structure for TeBr4. What is the molecule's
SOLVED Draw the Lewis structure for the tellurium tetrabromide (TeBr

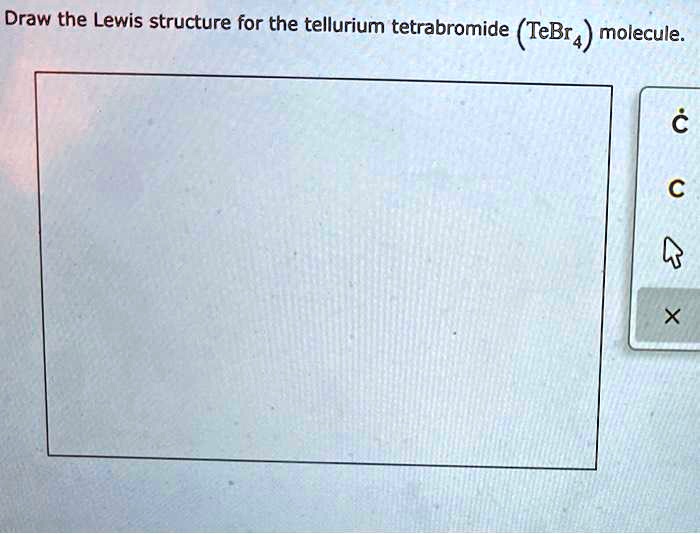
Draw the Lewis structure for the tellurium tetrabromide (TeBr_4

12+ Tebr4 Molecular Geometry Full GM
What is the molecular geometry of TeBr4? Quora
Web Molecular Formula Brte Average Mass 447.216 Da Monoisotopic Mass 445.579529 Da Chemspider Id 74282 More Details:
Each Fluorine Atom Has Three Lone Pairs, While The Tellurium Atom Has One Lone Pair.
Х This Problem Has Been Solved!
There Are 4 Single Bonds Between The Tellurium Atom (Te) And Each Bromine Atom (Br).
Related Post: