Draw The Lewis Structure For The Xenon Difluoride Molecule
Draw The Lewis Structure For The Xenon Difluoride Molecule - The lewis structure of xef6 shows that xenon is the central atom, surrounded by six fluorine atoms. Web what is the lewis structure of xef2? The electronegativity of f is 4.0. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. Web xeo2f2 is a chemical formula for xenon dioxy difluoride. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web xenon difluoride formula : A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. (2) draw single bonds between bonded atoms. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Find the total valence electrons in xef2 molecule in order to find the total valence electrons in xef2 (xenon. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. (5 points) draw the lewis structure for xenon difluoride. It shows xenon (xe) as the central atom bonded to two fluorine (f) atoms. Web the xef6 lewis structure refers to the arrangement of atoms and electrons in a molecule of xenon hexafluoride.xenon hexafluoride is. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. However, in the excited state, its configuration will change to 5s 2 5p 5 5d 1. You'll. I also go over hybridization, shape and bond angle. It’s ground state electronic configuration will be 5s 2 5p 6. Web the structure of xenon difluoride is illustrated below. Xef2 lewis structure and its properties are illustrated in this article. (5 points) draw the lewis structure for xenon difluoride. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Let’s draw and understand this lewis dot structure step. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw. Trigonal bipyramidal molecular geometry :. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Web to draw the xef2 lewis structure, follow these steps: The xenon atom (xe) is at the center and it is surrounded by 2 fluorine atoms (f). Find the total valence electrons in xef2 molecule in order to find the total valence. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (4 points)what is the molecular shape of xenon difluoride? Web in the hybridization of xenon difluoride, xenon (xe) is the central atom. (b) (2 points) c the electronegativity of xe is 2.6. (5 points) draw the lewis structure for xenon difluoride. Web xef2 lewis structure + molecular geometry chem101csub 3.77k subscribers subscribe 43 14k views 9 years ago chemistry learning made easy. The xenon atom (xe) is at the center and it is surrounded by 2 fluorine atoms (f). Xef2 lewis structure and its properties are illustrated in this article. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. (a) (2 points) in the space provided below, draw a lewis structure for the molecule xenon difluoride, xef2. It’s ground state electronic configuration will be 5s 2 5p 6. (2) draw single bonds between bonded atoms. (b) (2 points). Web in the hybridization of xenon difluoride, xenon (xe) is the central atom. It’s ground state electronic configuration will be 5s 2 5p 6. Web the xef6 lewis structure refers to the arrangement of atoms and electrons in a molecule of xenon hexafluoride.xenon hexafluoride is a compound composed of one xenon atom bonded to six fluorine atoms. Web a video. Xef2 lewis structure and its properties are illustrated in this article. (1) find the number of valence electrons in the molecule. Web xef2 lewis structure + molecular geometry chem101csub 3.77k subscribers subscribe 43 14k views 9 years ago chemistry learning made easy. However, in the excited state, its configuration will change to 5s 2 5p 5 5d 1. Let’s draw and understand this lewis dot structure step. (5 points) draw the lewis structure for xenon difluoride. This problem has been solved! Web drawing lewis structures for molecules with one central atom: Web what is the lewis structure of xef2? (4 points)what is the molecular shape of xenon difluoride? The xenon atom also holds 3 lone pairs of electrons. Web in xef2 molecule, two fluorine atoms are arranged symmetrically on the outside with the central atom xenon in the middle. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. It is one of those rare compounds which involve noble gases despite their strong stability. Web to draw the xef2 lewis structure, follow these steps: The lewis structure of xef6 shows that xenon is the central atom, surrounded by six fluorine atoms.
The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
xenon difluoride Overview, Structure, Properties & Uses

Xenon difluoride, 99.5+, Thermo Scientific Chemicals Fisher Scientific

XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Hello Guys! Today we are going to look at the Lewis Structure of XeF2

XeO2F2 Lewis Structure How to Draw The Lewis Structure for XeO2F2

Number of Lone Pairs and Bonding Pairs for XeF2 (Xenon difluoride
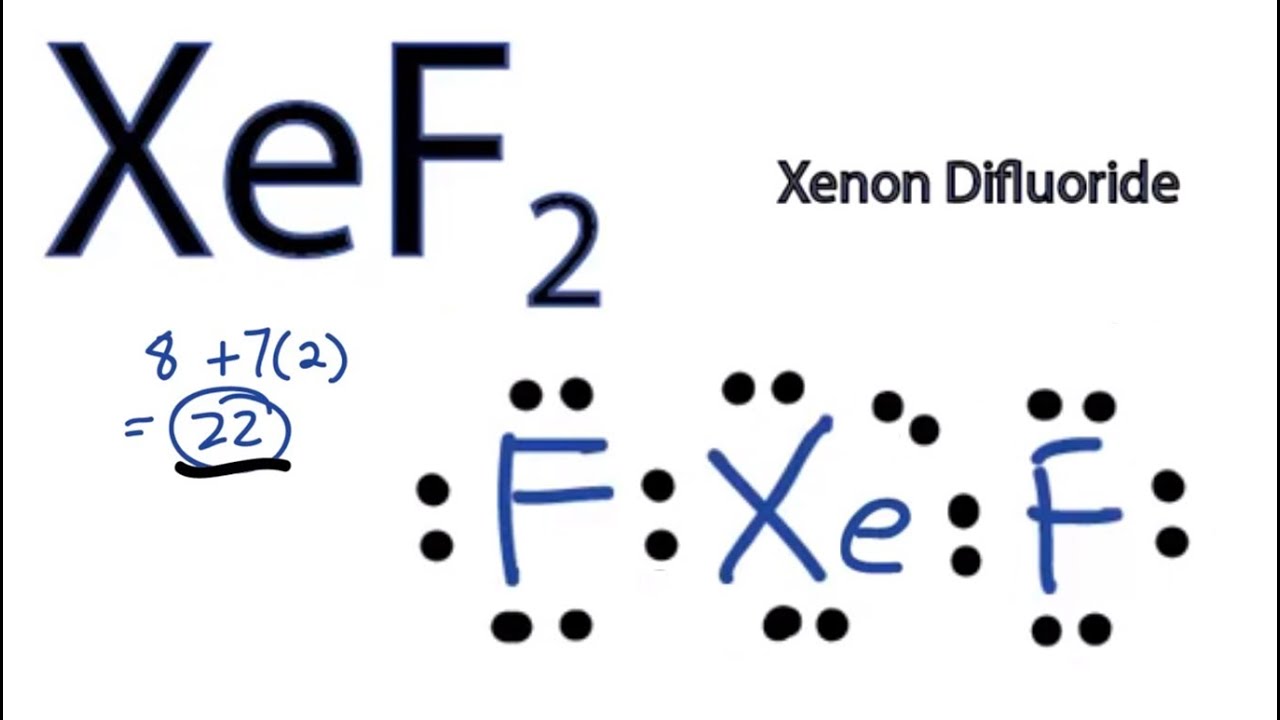
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube
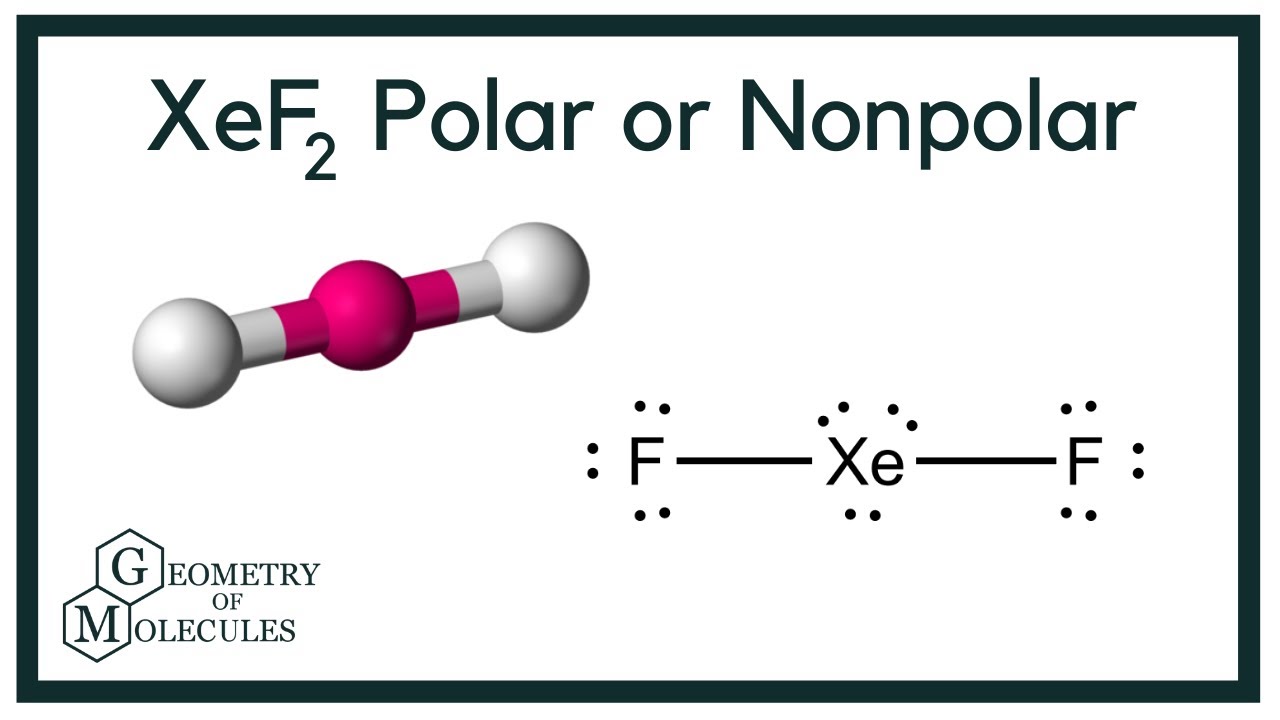
Xenon Difluoride Shape Draw Easy

Xenon Difluoride Molecular Structure Isolated on Black. 3d Illustration
It’s Ground State Electronic Configuration Will Be 5S 2 5P 6.
I Also Go Over Hybridization, Shape And Bond Angle.
Each Fluorine Atom Forms A Single Bond With The.
Web Xef2 Lewis Structure Is The Abbreviation Of Xenon Difluoride.
Related Post: