Draw The Lewis Structure For Xef2
Draw The Lewis Structure For Xef2 - For the central xenon atom: Xenon has eight valence electrons, and each fluorine atom has seven valence electrons, so the total number of valence electrons is 22. Web use these steps to correctly draw the xef 2 lewis structure: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. There are a total of 5 electron pairs in this lewis structure. Web lewis structure is based on the octet rule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Ii the number of lone pairs the number of single bonds the number of double bonds = 2. In the ammonia molecule a lone pair on nitrogen resides in a (n) a. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required let’s discuss each step in more detail. If there are more electrons than it, then that compound donates the electron. Sp3 hybrid orbital show transcribed image text here’s the best way to solve it. In the ammonia molecule a lone pair on nitrogen resides in a (n) a. Xenon (xe) is in group 18 of the periodic table and has 8 valence electrons. Web science chemistry chemistry. Web lewis structure is based on the octet rule. Sp3 hybrid orbital show transcribed image text here’s the best way to solve it. Web use these steps to correctly draw the xef 2 lewis structure: Web xef2 lewis structure is the abbreviation of xenon difluoride. Draw the lewis structure for xef2 a) how many groups (atoms and lone pairs) surround. Xenon (xe) can have more than 8. The central xenon atom a. Fluorine (f) is in group 17 and has 7 valence electrons each. We use dots to represent outer shell electrons and lines to represent the bond type. For the central xenon atom: The lewis structure for xef 2 requires you to place more than 8 valence electrons on xe. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. As we know xenon lies in. In the ammonia molecule a lone pair. Web by using the following steps, you can easily draw the lewis structure of xef 2: Obeys the octet rule b. Fluorine (f) is in group 17 and has 7 valence electrons each. Web the lewis structure of xenon difluoride (xef2) consists of a xenon (xe) atom at the center. I quickly take you through how to draw the lewis. For the central xenon atom: What is the electronic geometry of this molecule (look at atoms and lone pairs)? Obeys the octet rule b. Web chemistry chemistry questions and answers draw a lewis structure for xef2 and answer the following questions based on your drawing. Web chemistry chemistry questions and answers draw a lewis structure for xef2 and answer the. Web © 2023 google llc for the xef2 structure use the periodic table to find the total number of valence electrons for the xef2 molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Xef2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Determine the total number of valence. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. Web lewis structure is based on the octet rule. Web drawing the lewis structure for xef 2. As we know xenon lies in. Web © 2023 google llc for the xef2 structure use the periodic table to. Web science chemistry chemistry questions and answers draw the lewis structure for xef2 in the window below and then answer the questions that follow. Web © 2023 google llc for the xef2 structure use the periodic table to find the total number of valence electrons for the xef2 molecule. While selecting the atom, always put the least electronegative atom at. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Obeys the octet rule b. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. We use dots to. Obeys the octet rule b. Determine the total number of valence electrons. Draw the lewis structure for xef2 a) how many groups (atoms and lone pairs) surround the central oxygen? Sp3 hybrid orbital show transcribed image text here’s the best way to solve it. Web science chemistry chemistry questions and answers draw the lewis structure for xef2 in the window below and then answer the questions that follow. The central xenon atom a. Web the lewis structure of xenon difluoride (xef2) consists of a xenon (xe) atom at the center. Web by using the following steps, you can easily draw the lewis structure of xef 2: For the xef2 structure use the periodic table to find the total number of valence electron. Find the total valence electrons in xef2 molecule in order to find the total valence electrons in xef2 (xenon difluoride) molecule, first of all you should know the valence electrons present in xenon atom as well as fluorine atom. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). We use dots to represent outer shell electrons and lines to represent the bond type. Determine the total number of valence electrons in xef2 by adding the valence electrons of each atom. Web use these steps to correctly draw the xef 2 lewis structure: Hydrogen (h) only needs two valence electrons to have a full outer shell. Sp4 a draw a lewis structure for the ammonia molecule (nh3) and complete the following statement:![Molecular geometry of XeF2 [with video and free study guide]](https://aceorganicchem.com/chemistry/wp-content/uploads/2023/06/XeF2-lewis.jpg)
Molecular geometry of XeF2 [with video and free study guide]

XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 (Xenon

XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
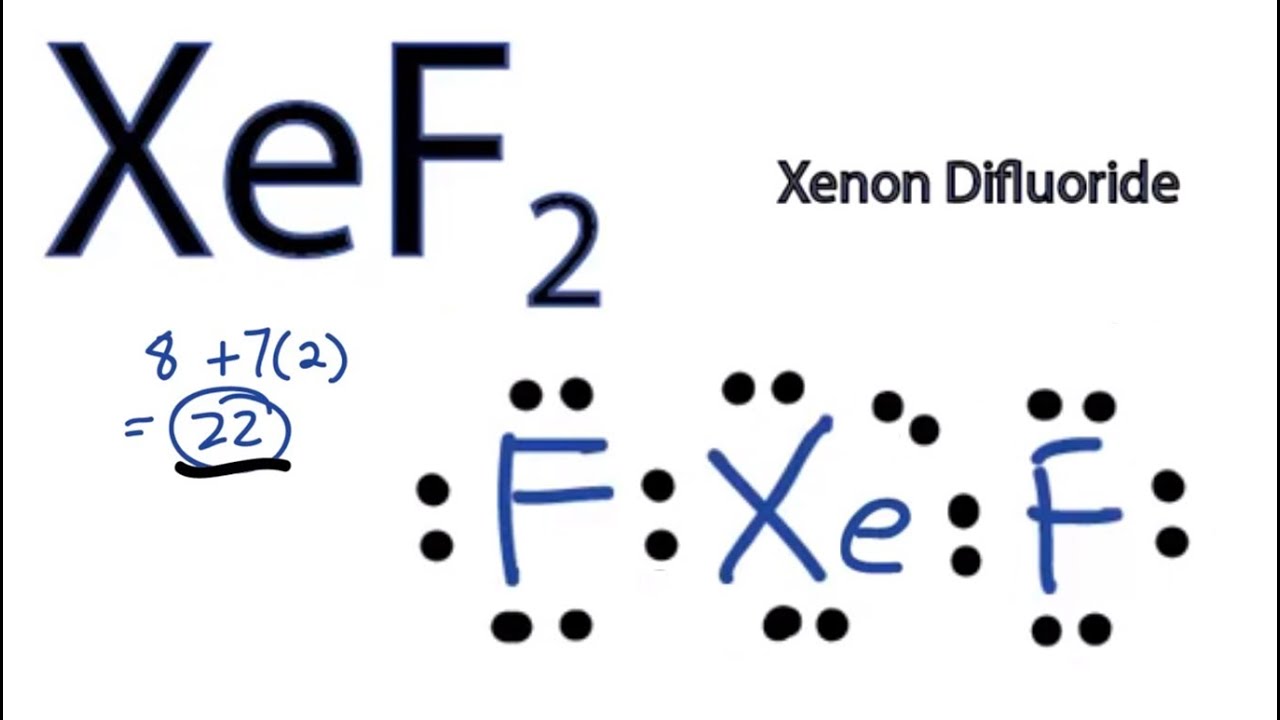
XeF2 Lewis Structure How to Draw the Lewis Structure for XeF2 YouTube

The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon

Hello Guys! Today we are going to look at the Lewis Structure of XeF2

36+ Xef2 Lewis Structure Molecular Geometry Image GM

Xef2 Lewis Structure Lone Pairs Drawing Easy
[Solved] Identify the correct Lewis structure for XeF2. O F=Xe=F O

The Lewis Structure Of Xef2 Understanding The Bonding Of Xenon
Determine The Total Number Of Valence Electrons:
Xenon Has Eight Valence Electrons, And Each Fluorine Atom Has Seven Valence Electrons, So The Total Number Of Valence Electrons Is 22.
In The Ammonia Molecule A Lone Pair On Nitrogen Resides In A (N) A.
D) What Are The Bond Angles?
Related Post: