Draw The Lewis Structure Of Ch3Br
Draw The Lewis Structure Of Ch3Br - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web check me out: Web to draw the ch3br lewis structure, follow these steps: It has only one carbon atom. For the ch3br molecule (c is the central atom), draw the correct lewis structure and select all polar covalent bonds. Web this is the total number of electrons that must be used in the lewis structure. This widget gets the lewis structure of chemical compounds. Web to properly draw the ch 3 br lewis structure, follow these steps: Web by using the following steps, you can easily draw the lewis structure of ch 3 br. Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. This widget gets the lewis structure of chemical compounds. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. • we will begin by calculating the total number of valence electrons in this. This widget gets the lewis structure of chemical compounds. Draw the molecule by placing atoms on the grid and connecting them with bonds. This is the ch3br lewis structure. The bond angle around each carbon atom is 109.5°.4. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the. Ch 3 br lewis structure For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The bond length between the carbon and hydrogen atoms is 1.09 å.5. There is a single bond between the carbon (c) & bromine. In this post, we discussed the method to construct the ch3br lewis structure. Web check me out: The type of hybridization around the br atom is sp3.3. The bond length between the carbon and hydrogen atoms is 1.09 å.5. You can intuitively draw any lewis structure on your computer or mobile device. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web to properly draw the ch 3 br lewis structure, follow these steps: For the ch3br structure use the periodic table to find the total number of valence electrons. It has only one carbon atom. You can intuitively. There are 3 lone pairs on the bromine atom (br). O + 2 (f) = of 2. This widget gets the lewis structure of chemical compounds. There is a single bond between the carbon (c) & bromine (br) atom as well as between the carbon (c) and hydrogen (h) atoms. For the ch3br molecule (c is the central atom), draw. O + 2 (f) = of 2. The central atom is carbon, which is bordered on four terminals with one bromine atom, three. Web the lewis structure for ch3br is shown below: Web drawing lewis structures for molecules with one central atom: There are 3 lone pairs on the bromine atom (br). It is a chemical formula for bromomethane. Find more chemistry widgets in wolfram|alpha. In the case of ch3br, carbon being a group. #1 draw a rough sketch of the structure first, determine the total number of valence electrons Web the lewis structure for ch3br is shown below: In order to draw the lewis. For the ch3br structure use the periodic table to find the total number of valence electrons. For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. Select the center atom (h is always outside). There are 3 lone pairs on the bromine atom (br). The bond length between the carbon and bromine. The bond length between the carbon and hydrogen atoms is 1.09 å.5. Find more chemistry widgets in wolfram|alpha. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web to properly draw the ch 3 br lewis structure, follow these steps: For ch3br, the carbon has four valence electrons, each hydrogen has one, and bromine has seven. The first step is to sketch the molecular geometry of the ch3br molecule, to calculate the lone pairs of the electron in the central carbon, terminal bromine, and terminal hydrogen atom; The second step is to calculate the ch3br hybridization, and the third step is to give. Web hello guys, welcome back to our channel, and in today's video, we share our quick and easy method to determine the lewis structure of the ch3br molecule. The type of hybridization around the br atom is sp3.3. When the question is first opened, a box with a. Draw the lewis structure of ch3br draw the lewis structure of ch3br here’s the best way to solve it. Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. The bond angle around each carbon atom is 109.5°.4. Web chemistry chemistry questions and answers draw the lewis structure of ch3br this problem has been solved! Determine the central atom in the molecule. The bond length between the carbon and bromine. Web see the big list of lewis structures. • we will begin by calculating the total number of valence electrons in this molecule. Web the lewis structure for ch3br is shown below: #1 draw a rough sketch of the structure first, determine the total number of valence electrons
How to draw CH3Br Lewis Structure? Science Education and Tutorials

CH3Br (Bromomethane) Molecular Geometry, Bond Angles YouTube

How to draw CH3Br Lewis Structure? Science Education and Tutorials
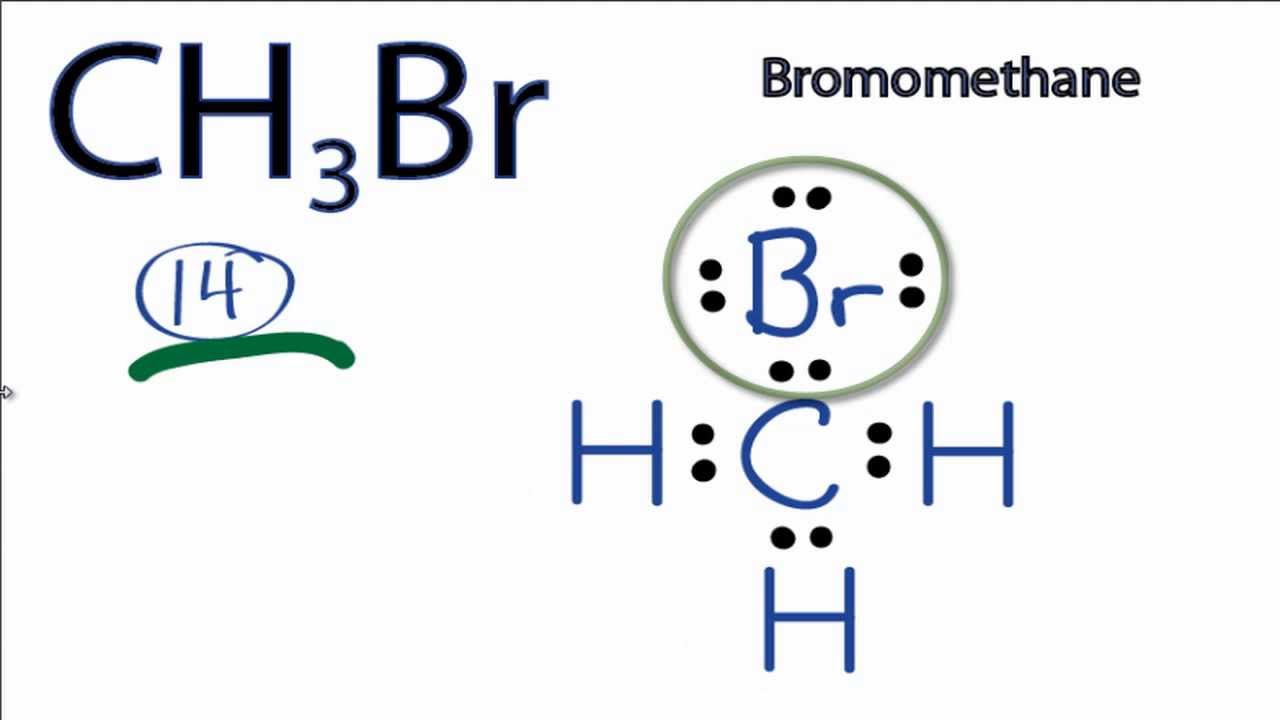
CH3Br Lewis Structure How to Draw the Lewis Structure for CH3Br

CHBr3 Molecular Geometry Science Education and Tutorials

CH3Br Lewis Structure (Bromomethane) YouTube

CH3Br Lewis Structure, Geometry, Hybridization, and Polarity

draw the lewis structure of ch3br sectionalviewsengineeringdrawing

CH3Br Lewis Structure, Geometry, Hybridization, and Polarity
[Solved] Draw the Lewis structure of bromoform (CH3Br). a. What is the
Web By Using The Following Steps, You Can Easily Draw The Lewis Structure Of Ch 3 Br.
Web Ch3Br Lewis Structure Has A Carbon Atom (C) At The Center Which Is Surrounded By Three Hydrogen Atoms (H) And One Bromine Atom (Br).
Web To Draw The Ch3Br Lewis Structure, Follow These Steps:
In The Case Of Ch3Br, Carbon Being A Group.
Related Post: