Draw The Lewis Structure Of Nbr3
Draw The Lewis Structure Of Nbr3 - Web concept explainers question make sure to draw all bonds and add all lone pair electrons. Web how to draw nbr3 lewis structure? Web chemistry chemistry questions and answers draw the lewis structure for nbr3 molecule. Web by using the following steps, you can easily draw the lewis structure of nbr 3. Nitrogen (n) has 5 valence electrons and bromine (br) has 7 valence. Nbr3 molecule has an overall count of valence electrons is 26. The central atom is nitrogen, which is bordered on three terminals with bromine atoms (. While selecting the atom, always put the least electronegative atom at the center. Question 4 2 pts draw a lewis structure for the nbr3 molecule. #2 mark lone pairs on the atoms. Question 4 2 pts draw a lewis structure for the nbr3 molecule. Find the total valence electrons in nbr3. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. This problem has been solved! Web in the nbr 3 lewis structure, there are three single bonds around. Number of unshared electron pairs around the central atom d. Web nbr3 lewis structure (nitrogen tribromide) | how to draw the lewis dot structure for nbr3 geometry of molecules 2.47k subscribers subscribe 528 views 1 year ago lewis structure. Here, the given molecule is nbr3 (nitrogen tribromide). Find out the central atom. More this problem has been solved! We are asked to write the lewis structure of k r cl 2, which is a noble gas, a noble gas with eight electrons and a chlorine with seven electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nbr3 lewis structure, or you can also watch this short 2 minute video). #2. Key points to consider when drawing the nbr3 electron dot structure. Identify the shape of the molecule. Web the first step is to sketch the molecular geometry of the nbr3 molecule, to calculate the lone pairs of the electron in the central nitrogen atom; Web by using the following steps, you can easily draw the lewis structure of nbr 3.. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Identify the shape of the molecule. If you want to know the steps of drawing the nbr3 lewis dot structure, then visit this article: Web 82k what is the octet rule? Total number of valence electrons from all atoms b. Draw the lewis structure of nbr3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The interatomic chemical bonds in a molecule are shown in lewis dot structure diagrams, also known a. Web this problem has been solved! Draw the lewis dot structure for ch3cho. Step by step solved in 3 steps with 4 images see solution check out a sample q&a here knowledge booster learn more about theories of bonding Draw the lewis dot structure for ch3cho. #1 first draw a rough sketch. Draw the lewis structure of nbr3. Draw the lewis structure of nbr 3. Web this problem has been solved! The interatomic chemical bonds in a molecule are shown in lewis dot structure diagrams, also known a. Web by using the following steps, you can easily draw the lewis structure of nbr 3. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step. Each bromine atom has three lone pairs, and the nitrogen atom has one lone pair. Here, the given molecule is nbr3 (nitrogen tribromide). Web how to draw nbr3 lewis structure? See the rules for drawing lewis structure and its dependency on the periodic table. More this problem has been solved! Web how to draw nbr3 lewis structure? We are asked to write the lewis structure of k r cl 2, which is a noble gas, a noble gas with eight electrons and a chlorine with seven electrons. Find the total valence electrons in nbr3. Web 6 steps to draw the lewis structure of nbr3 step #1: Draw the lewis dot. Web how to draw nbr3 lewis structure? You'll get a detailed solution from a subject matter expert. #2 mark lone pairs on the atoms. Expert solution trending now this is a popular solution! This problem has been solved! Web nbr3 lewis structure (nitrogen tribromide) | how to draw the lewis dot structure for nbr3 geometry of molecules 2.47k subscribers subscribe 528 views 1 year ago lewis structure. This compound has 2 central atoms what specific type of covalent bond is there between the c and o in your structure above? Connect the atoms to each other with single bonds to form a “skeleton structure.”. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Explain the steps that you took in creating the lewis dot structure for this compound. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See the rules for drawing lewis structure and its dependency on the periodic table. If you want to know the steps of drawing the nbr3 lewis dot structure, then visit this article: Web 6 steps to draw the lewis structure of nbr3 step #1: Number of unshared electron pairs around the central atom d.
Nbr3 Lewis Structure Draw Easy

What is the Lewis structure of NBr3? YouTube
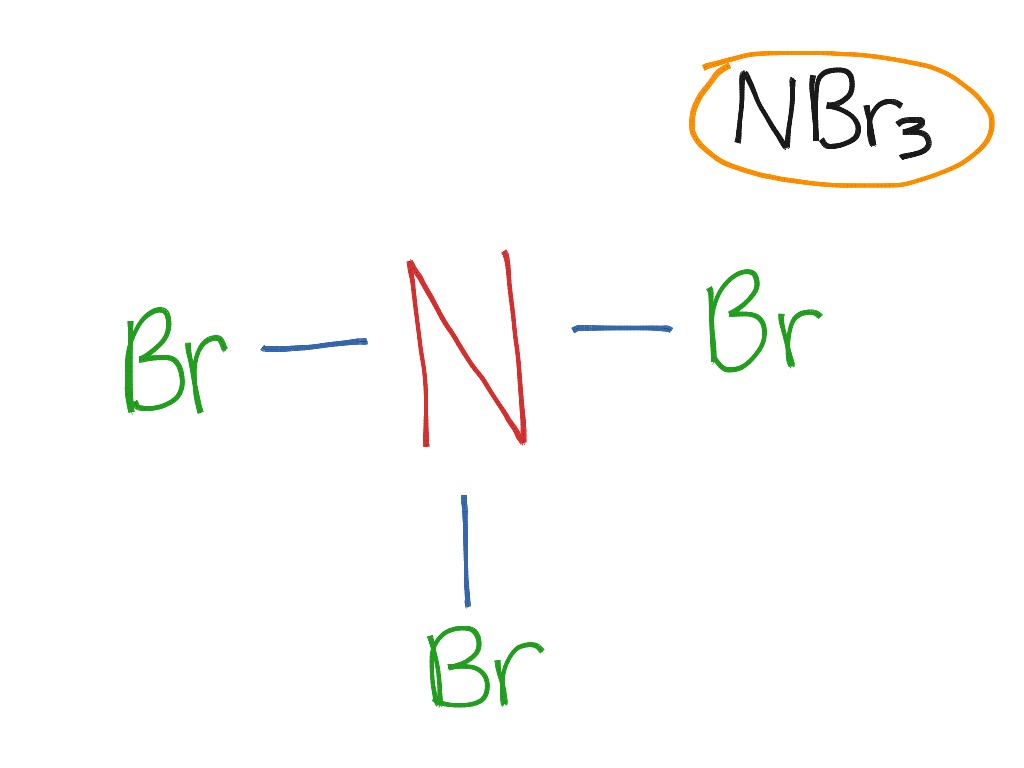
Covalent bond NBr3 Science ShowMe

NBr3 Covalent Bond Science ShowMe
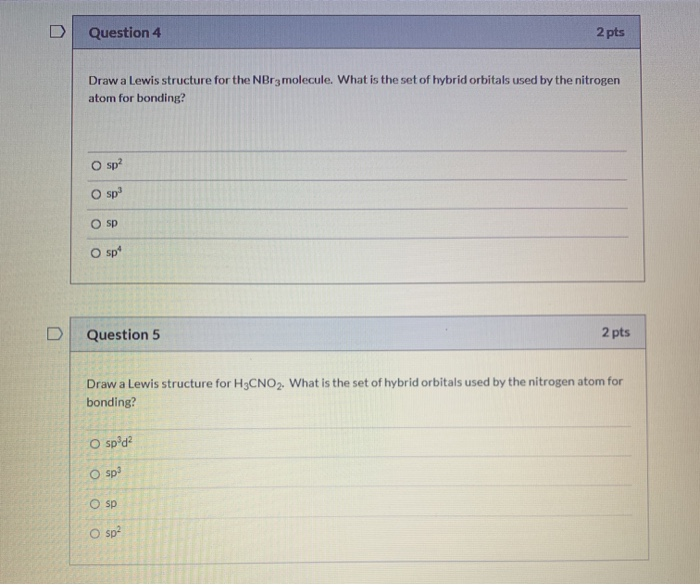
Solved Question 4 2 pts Draw a Lewis structure for the NBr3

NBr3 lewis Structure (Nitrogen Tribromide) How to Draw the Lewis Dot

NBr3 Lewis StructureLewis Structure of NBr3 (Nitrogen Tribromide
Solved Give the Lewis Dot Structure for NBr_3. Give the

So far, we’ve used 26 of the NBr3 Lewis structure’s total 26 outermost

How to Draw the Lewis Dot Structure for NBr3 Nitrogen tribromide YouTube
Web The First Step Is To Sketch The Molecular Geometry Of The Nbr3 Molecule, To Calculate The Lone Pairs Of The Electron In The Central Nitrogen Atom;
#1 First Draw A Rough Sketch.
Number Of Shared Electron Pairs Around The Central Atom C.
Find Out The Central Atom.
Related Post: