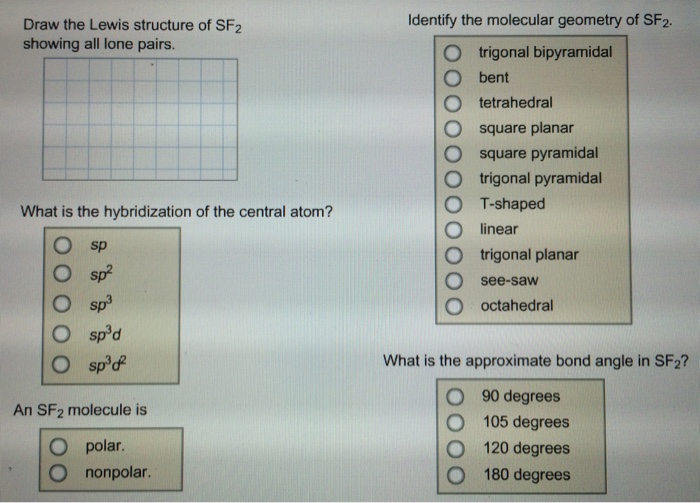
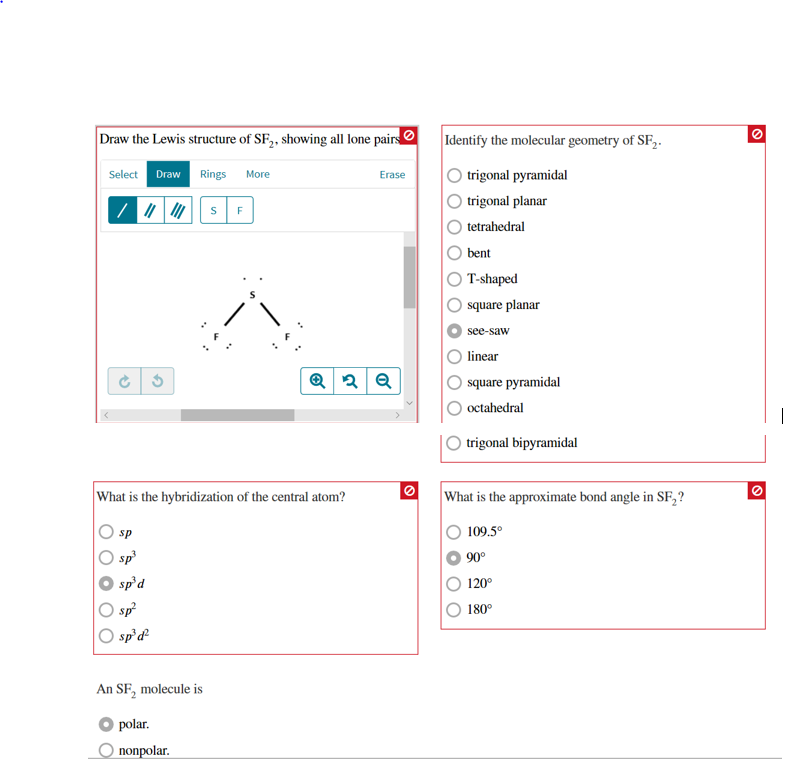
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs - Identify the molecular geometry of sf2. In sf2, sulfur (s) is the central atom. Identify the molecular geometry of sf2. Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. The lewis structure for sf2 (sulfur difluoride) involves a total of 20 valence electrons. In this question firstly we have to write down the lewis structure of sf2. Identify the molecular geometry of sf2. What is the approximate bond angle in sf2? Web draw the lewis structure of sf2, showing all lone pairs. Therefore, the total number of valence electrons in sf2 is: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. What is the hybridization of the central atom? Web drawing lewis structures for molecules with one central atom: Web draw the lewis structure of sf2, showing all lone pairs. Number of valence electrons in two f atom = 2 (7) =. Web to summarise this blog, we can say that, sf2 has a simple lewis structure in which the sulphur atom is in the centre forming single bonds with both the fluorine atoms. Web chemistry questions and answers. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web sulfur. Identify the molecular geometry of sf2. Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. Identify the molecular geometry of sf2. Electrons used in bonding = 4 Web draw the lewis structure of sf2, showing all lone pairs. Web drawing lewis structures for molecules with one central atom: Web draw the lewis structure of sf2, showing all lone pairs. With a molar mass of 70.062 g/mol, this compound is made up of one sulfur atom and two fluoride atoms. In sf2, each fluorine atom already has an octet (8 valence electrons). Count the total number of valence electrons: Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury (||) fluoride. Web question draw the lewis structure of sf2. Include all the lone pairs. From these geometries, decide on the omo bond angle, the average no bond order,. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury (||) fluoride. Web drawing lewis structures for molecules with one central atom: With a molar mass of 70.062 g/mol, this compound is made up of one sulfur atom and two fluoride atoms. Sulfur has 6 valence electrons, and each fluorine atom has 7. What is the hybridization of the central atom? What is the hybridization of the central atom? The central atom of sulfur difluoride gets attached to the fluorine, so let's consider the solution here. Sulfur (s) has 6 valence electrons and each fluorine (f) has 7 valence electrons. Web draw the lewis structure of sf2, showing all lone pairs. Web place the remaining valence electron pairs on the central atom. Web drawing lewis structures for molecules with one central atom: What is the hybridization of the central atom? Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. In sf2, each fluorine atom already has an octet (8 valence electrons). Place the remaining 16 electrons as lone pairs on the sulfur atom. 6 + 2(7) = 20 valence electrons step 2/5 2. Web drawing lewis structures for molecules with one central atom: What is the hybridization of the central atom? Web question draw the lewis structure of sf2. The central atom of sulfur difluoride gets attached to the fluorine, so let's consider the solution here. Draw the lewis structure of sf2, showing all lone pairs. Erase q2 q identify the molecular geometry of sf2. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. There are two lone pairs. For the sf2 structure use the periodic table to find the total number of valence electrons for. Web chemistry chemistry questions and answers draw the lewis structure of sf2. Identify the molecular geometry of sf2. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Draw the lewis structure of sf2, showing all lone pairs. This gives the molecule a bent shape. Identify the molecular geometry of sf2. Draw the lewis structure of sf2. Instant answer step 1/5 1. With a molar mass of 70.062 g/mol, this compound is made up of one sulfur atom and two fluoride atoms. Sulfur has 6 valence electrons, and each fluorine atom has 7 valence electrons. So this sulfur this will have the 6 valence electrons and the fluorine this will have the 7 valence electrons. Include all the lone pairs. What is the hybridization of the central atom? Web to summarise this blog, we can say that, sf2 has a simple lewis structure in which the sulphur atom is in the centre forming single bonds with both the fluorine atoms. Web chemistry chemistry questions and answers draw the lewis structure of sf2 showing all lone pairs.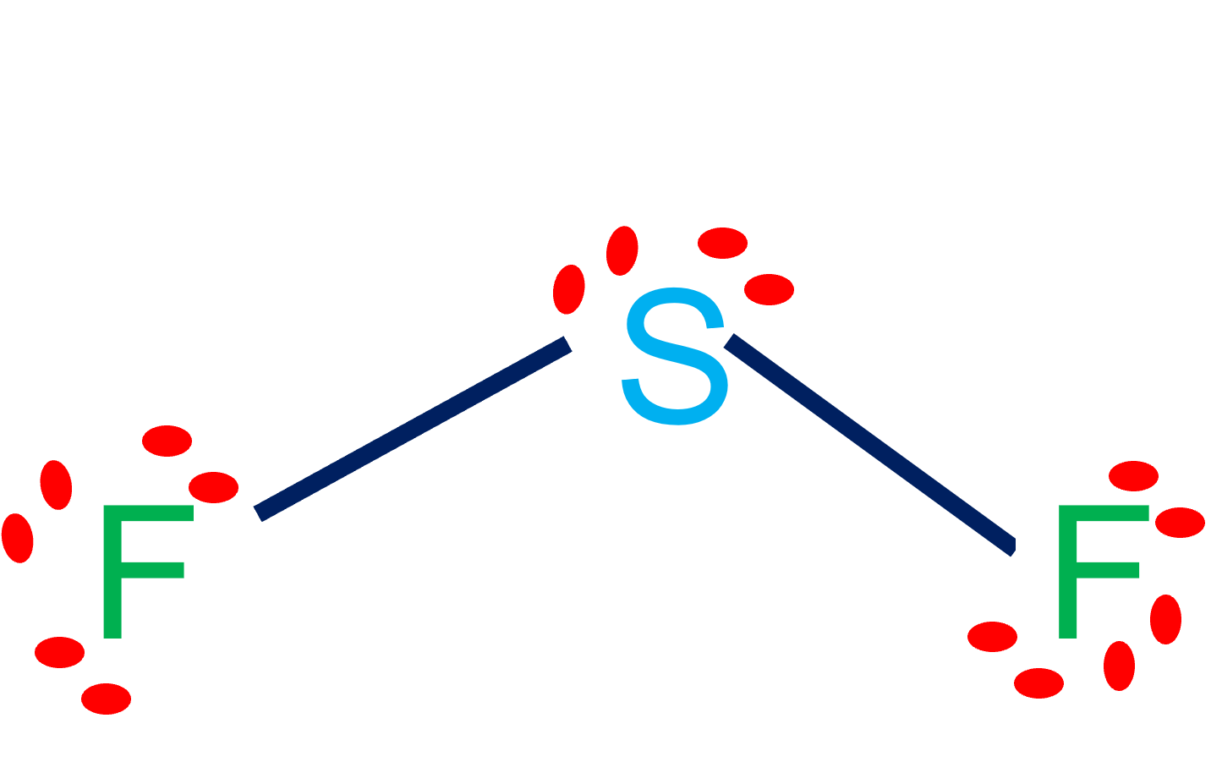
SF2 Lewis Structure & Molecular Geometry Simple Steps What's Insight
Draw the lewis structure of sf2 showing all lone pairs creativeprof
SOLVED Draw the Lewis structure of SF2, showing all lone pairs
Solved Draw The Lewis Structure Of SF2 Showing All Lone P...
Draw the lewis structure of sf2 showing all lone pairs torbucket

Draw the Lewis structure of SF2, showing all lone pairs. Identify the

Draw the lewis structure of sf2 showing all lone pairs jujaeazy

SF2 Lewis Structure How to Draw the Lewis Structure for SF2 YouTube

Draw the lewis structure of sf2 showing all lone pairs punchstart
Draw the lewis structure of sf2 showing all lone pairs punchstart
Include All The Lone Pairs.
Sp Sp2 Sp3 Sp3D Sp3D2 An Sf2 Molecule Is Polar, Nonpolar.
Web Sulfur Fluoride Is A Highly Unstable Inorganic Compound.
The Lewis Structure For Sf2 (Sulfur Difluoride) Involves A Total Of 20 Valence Electrons.
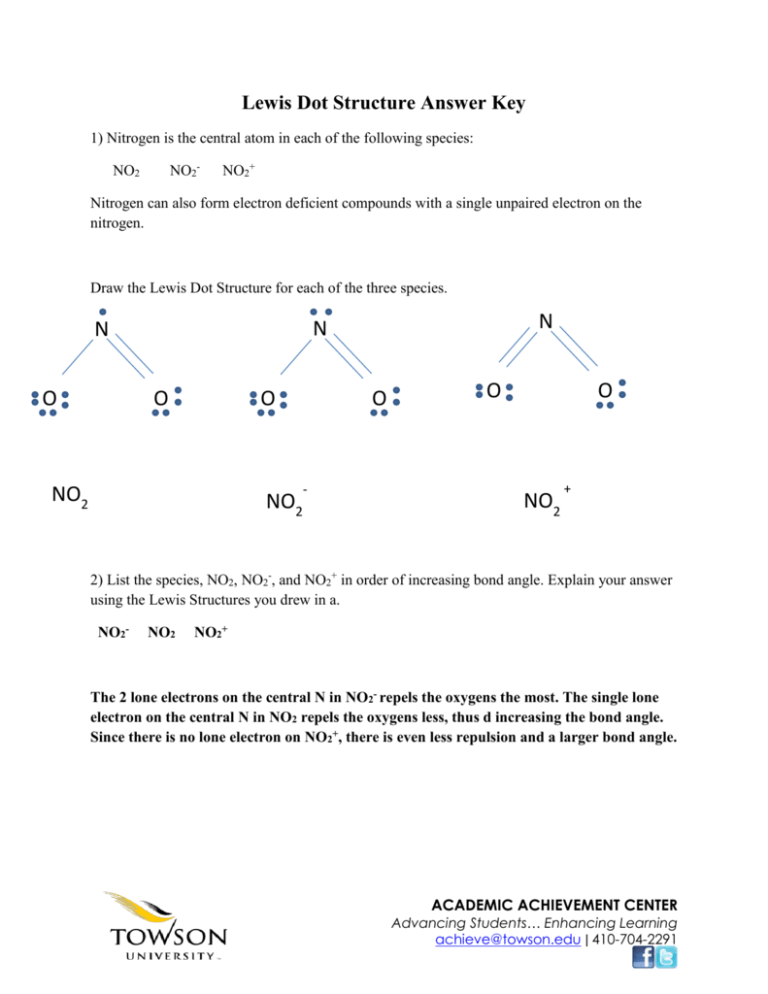
Related Post: