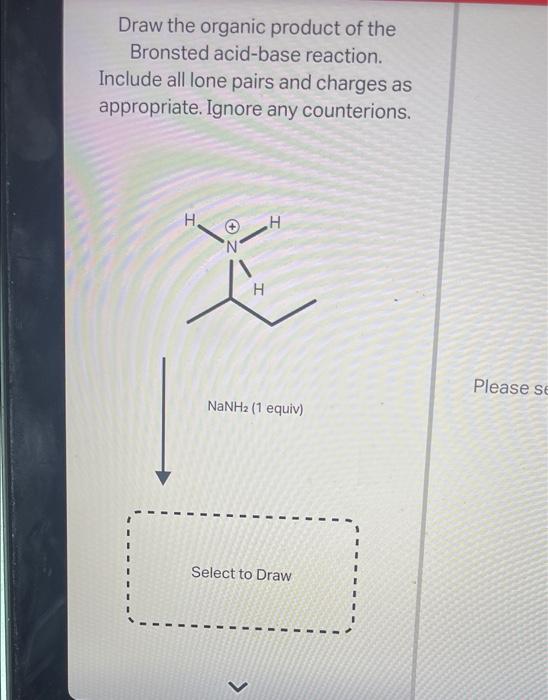
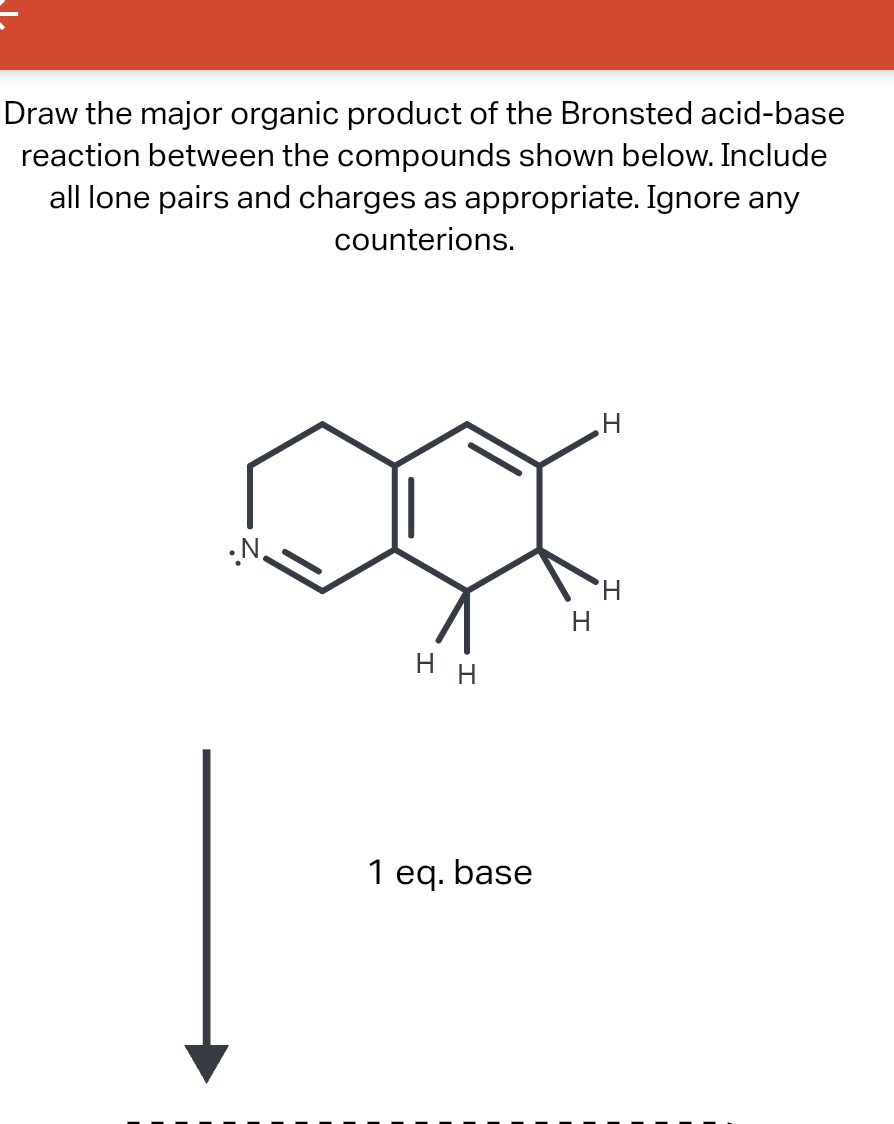
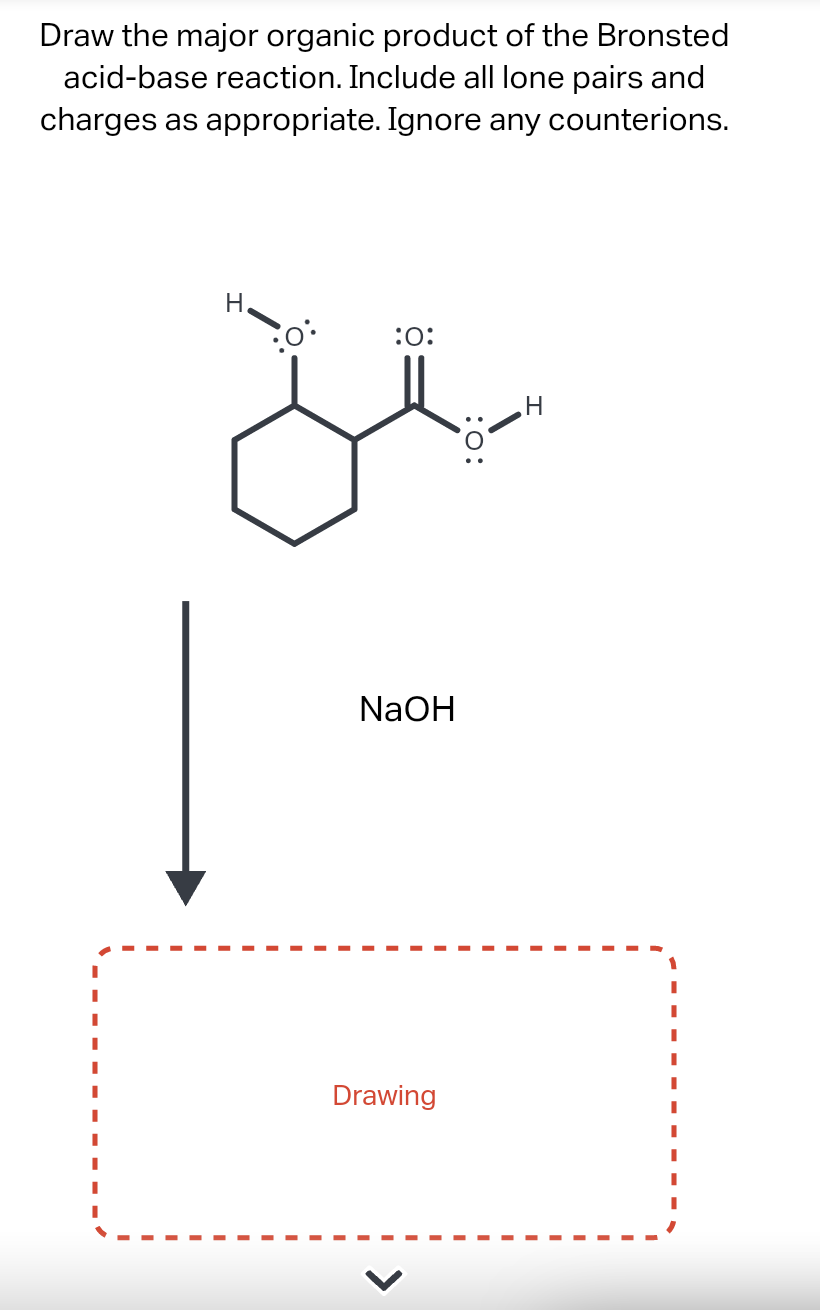
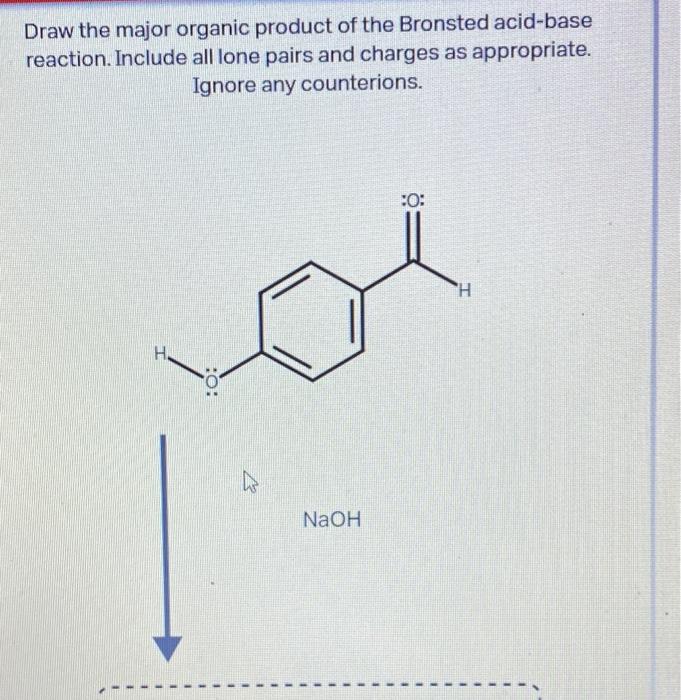
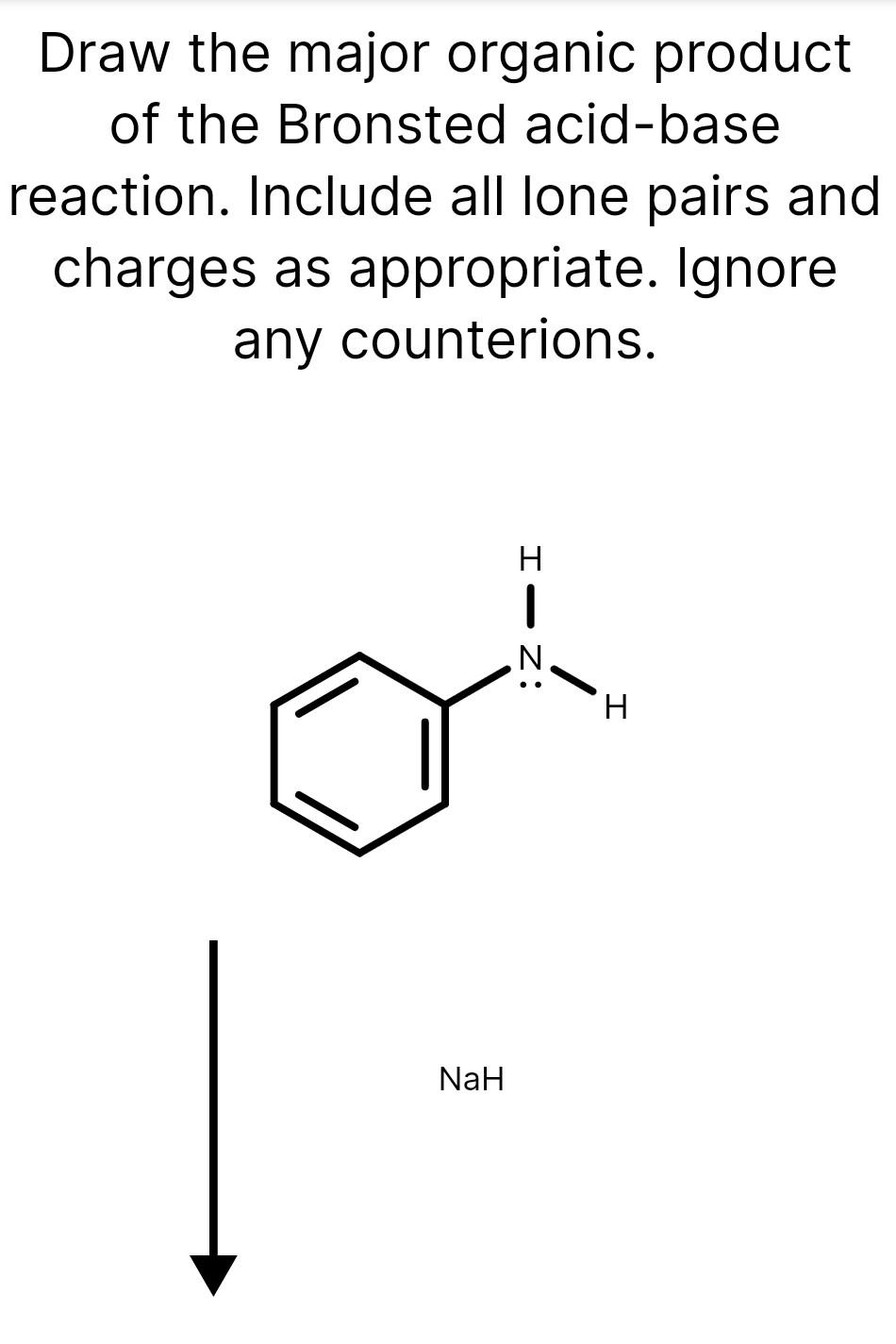
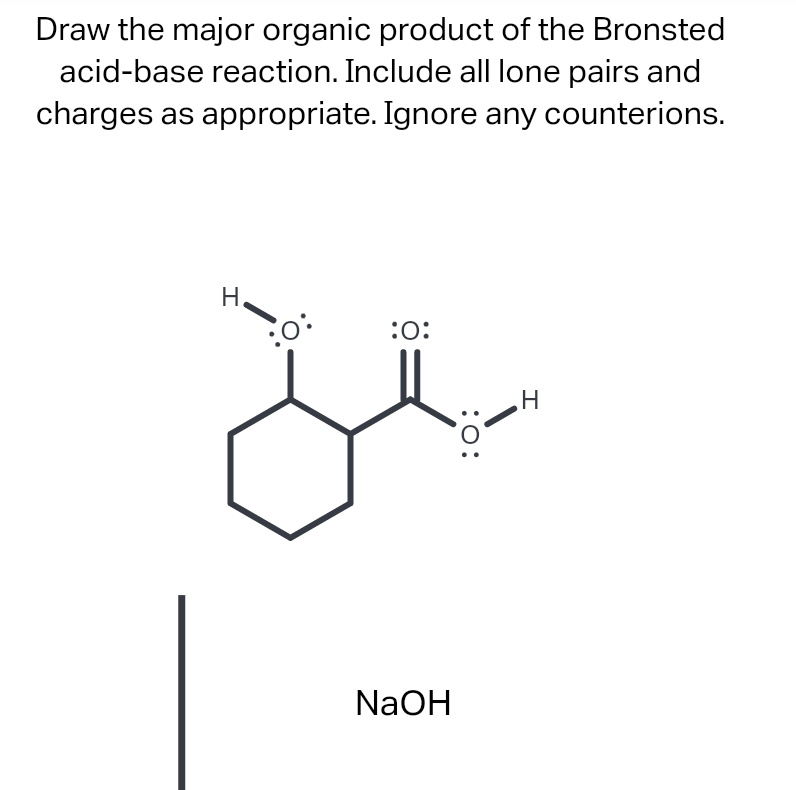
Draw The Major Organic Product Of The Bronsted Acid-Base Reaction
Draw The Major Organic Product Of The Bronsted Acid-Base Reaction - You do not have to consider stereochemistry. The product is water (the conjugate acid of hydroxide) and chloride ion (the conjugate base of hcl). Web organic chemistry i (cortes) 11: A proton is transferred from hcl, the acid, to hydroxide, the base. Web bookshelves organic chemistry map: Include all lone pairs and charges as appropriate. Base drawing h organic chemistry 9th edition isbn: This problem has been solved! Include all lone pairs and charges as appropriate. Include all lone pairs and charges as appropriate. Do not include lone pairs in your answer; You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The product would be alcohol. So in other words, it's going to lose a hydrogen. You have undoubtedly seen this. Include all lone pairs and charges as appropriate. Base drawing h organic chemistry 9th edition isbn: Top voted samantha nordin 8 years ago The product is water (the conjugate acid of hydroxide) and chloride ion (the conjugate base of hcl). There is a transfer of the proteins from benzoic acid to hydrocarbonate. The product would be alcohol. Include all lone pairs and charges as appropriate. So let's, really quickly, review what this. Include all lone pairs and charges as appropriate. You do not have to explicitly draw h atoms. A) for each compound show its conjugate base. Include all lone pairs and charges as appropriate. Base drawing h organic chemistry 9th edition isbn: Include all lone pairs and charges as appropriate. Top voted samantha nordin 8 years ago Include all lone pairs and charges as appropriate. Web organic chemistry i (cortes) 11: For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions: There is a transfer of the proteins from benzoic acid to hydrocarbonate. You have undoubtedly seen this. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions: You have undoubtedly seen this. Lone pairs have been left out. Questions tips & thanks want to join the conversation? Naoh select to fdit draw the organic product of. Include all lone pairs and charges as appropriate. This figure has two rows. Naoh select to fdit draw the organic product of. Include all lone pairs and charges as appropriate. There is a transfer of the proteins from benzoic acid to hydrocarbonate. Web bookshelves organic chemistry map: Include all lone pairs and charges as appropriate. This figure has two rows. Include all lone pairs and charges as appropriate. Include all lone pairs and charges as appropriate. You have undoubtedly seen this. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions: Lone pairs have been left out. A conjugate acid is the particle produced when a base accepts a proton. A proton is transferred from hcl, the acid, to hydroxide,. A conjugate acid is the particle produced when a base accepts a proton. There is a transfer of the proteins from benzoic acid to hydrocarbonate. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water. Include all lone pairs and charges as appropriate. Include all lone pairs and charges as appropriate. There is a transfer of the proteins from benzoic acid to hydrocarbonate. That's a mixture of two acids. This figure has two rows. You do not have to consider stereochemistry. They will not be considered in the grading. Include all lone pairs and charges as appropriate. This problem has been solved! Do not include lone pairs in your answer; So let's, really quickly, review what this. Web chemistry questions and answers. Include all lone pairs and charges as appropriate. B) rank the conjugate base in the order you would predict, from most to least stable. The product would be alcohol. Base drawing h organic chemistry 9th edition isbn:Solved Draw the organic product of the Bronsted acidbase
Solved Draw the major organic product of the Bronsted
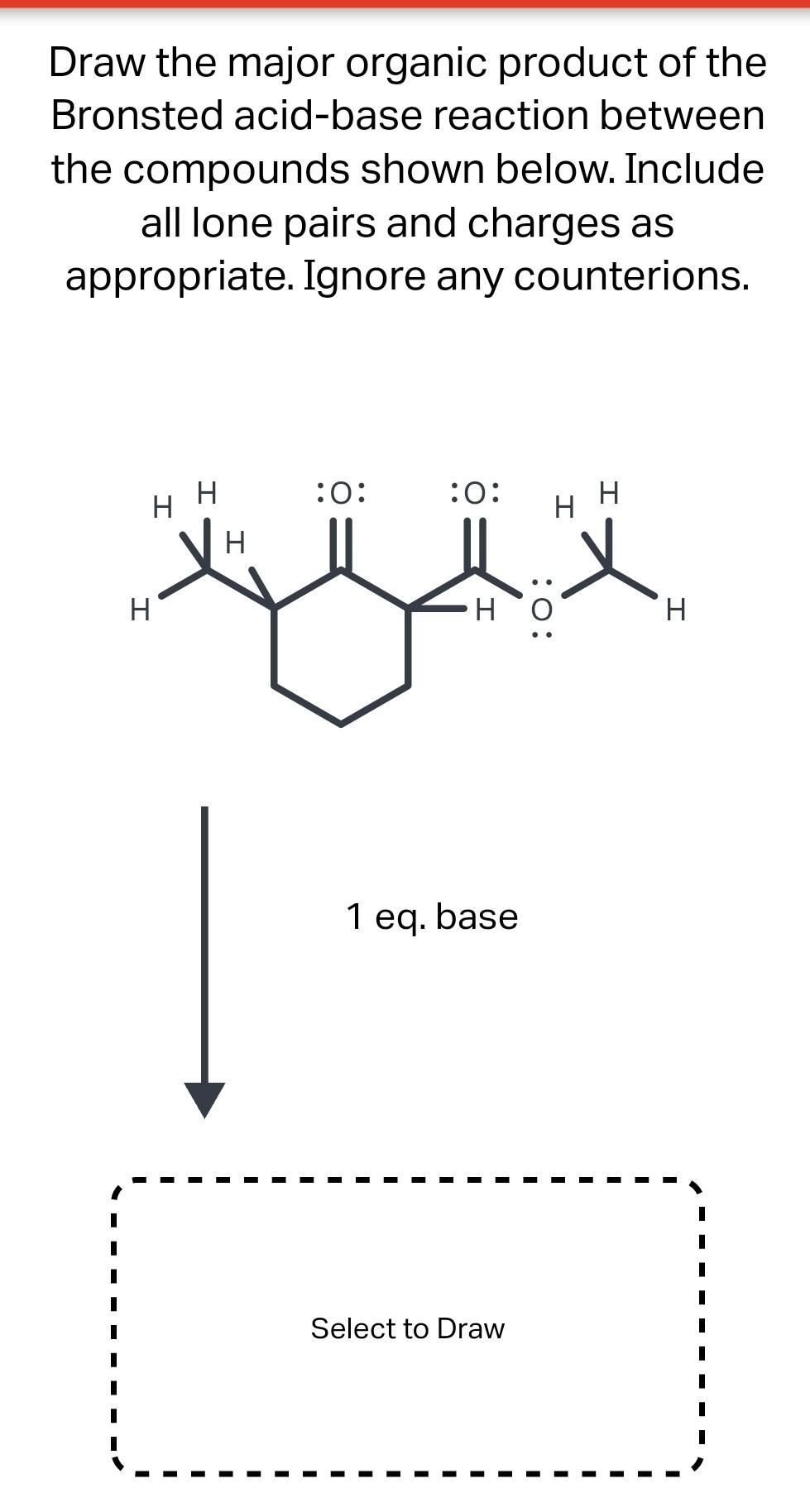
Solved Draw the major organic product of the Bronsted
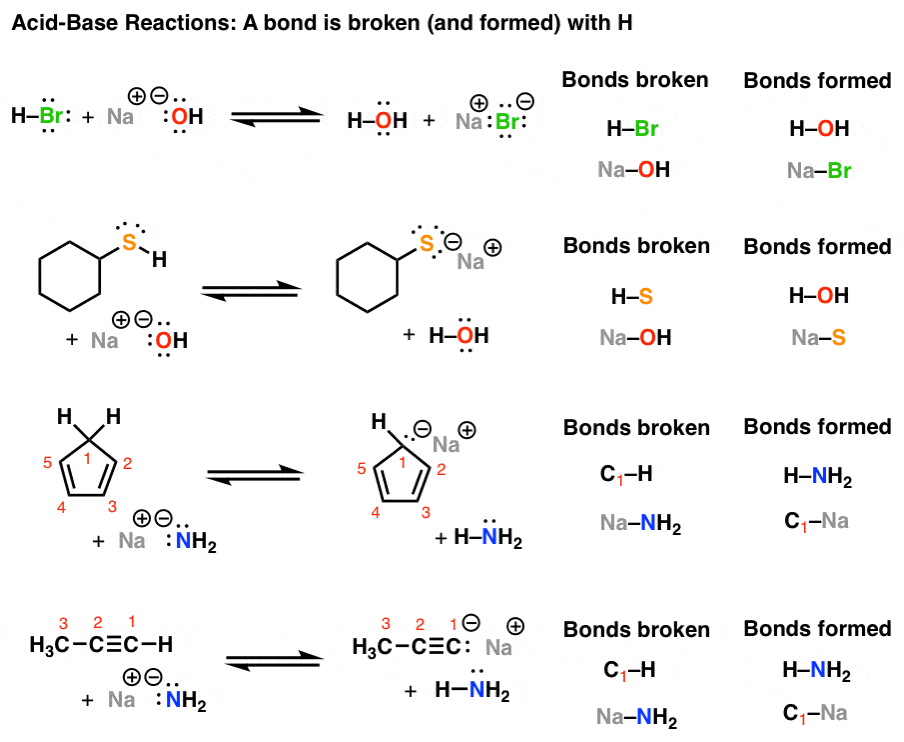
Introduction to AcidBase Reactions Master Organic Chemistry
Solved Draw the major organic product of the Bronsted
Solved Draw the major organic product of the Bronsted
Draw the major organic product of the Bronsted acidbase reaction
Solved Draw the organic product of the Bronsted acidbase
Solved Draw the major organic product of the Bronsted
[Solved] Draw the major organic product of the Bronsted acidbase
So In Other Words, It's Going To Lose A Hydrogen.
A Proton Is Transferred From Hcl, The Acid, To Hydroxide, The Base.
Include All Lone Pairs And Charges As Appropriate.
What Makes A Compound Acidic (Likely To Donate A Proton) Or Basic (Likely To Accept A Proton)?
Related Post: