Draw The Major Product For The Dehydration Of 2-Pentanol.
Draw The Major Product For The Dehydration Of 2-Pentanol. - Web dehydration reaction of secondary alcohol: Dehydration involves formation of the protonated alcohol, $$ roh^+_2 $$. In this case, the leaving group is water (h2o). Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. The dehydration mechanism for a tertiary alcohol is analogous to that shown above for a secondary alcohol. Ch3ch2ch(oh)ch2ch3 step 2/4 step 2: Determine the mechanism for the reaction. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Web draw an arrow pushing mechanism for the acid catalyzed dehydration of the following alcohol, make sure to draw both potential mechanisms. Web expert answer 100% (6 ratings) transcribed image text: Draw one structure per sketcher. Draw the structures, including hydrogen. Draw the structures of the two organic products of this reaction. The following are the steps involved. Addition to a carbonyl expand_more section: Study with quizlet and memorize flashcards. >the oh picks an acidic proton. Web dehydration reaction of secondary alcohol: Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. A guided inquiry 2nd edition isbn: Draw the structures of the two organic products of this reaction. > it then leaves as h2o leaving behind a positive charge. Determine the mechanism for the reaction. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Buy chemistry 10th edition isbn: Web predict the major product of acid catalysed dehydration: Draw the structures of the two organic products of this reaction. >the oh picks an acidic proton. Determine the mechanism for the reaction. Determine the mechanism for the reaction. Is used as dehydrating agent. >the oh picks an acidic proton. Part a draw the major product for the dehydration of 2.pentanol. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. A guided inquiry 2nd edition isbn: Draw one structure per sketcher. Web expert answer 100% (6 ratings) transcribed image text: Part a draw the major product for the dehydration of 2.pentanol. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Oh h2so4 major product + minor product draw the major product. Assume no rearrangement for the first two product mechanisms. Dehydration involves formation of the protonated alcohol, $$ roh^+_2 $$. Ch3ch2ch(oh)ch2ch3 step 2/4 step 2: Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. This problem has been solved! When more than one alkene product are possible, the favored product is usually the thermodynamically most stable alkene. Addition to a carbonyl expand_more section: If there was a rearrangement, draw the expected major product. Dehydration involves formation of the protonated alcohol, $$ roh^+_2 $$. This problem has been solved! Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. If there was a rearrangement, draw the expected major product. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! If there was a rearrangement, draw the expected major product. Draw the structures, including hydrogen atoms, of the two organic products of this reaction. Reaction mechanism for dehydration of alcohol involves a carbocation intermediate. Web dehydration reaction of secondary alcohol: Cengage learning expand_more chapter 1 : The following are the steps involved. Draw the structures of the two organic products of this reaction. The first step is the formation of a protonated alcohol. This reaction occurs through the elimination of water from the alcohol molecule. Ch3ch2ch(oh)ch2ch3 step 2/4 step 2: You do not have to consider stereochemistry. Is used as dehydrating agent. Dehydration of alcohol gives alkene as product. Assume no rearrangement for the first two product mechanisms. Reaction mechanism for dehydration of alcohol involves a carbocation intermediate. >it picks an acidic proton adjacent to the carbocation. In this case, the leaving group is water (h2o). If there was a rearrangement, draw the expected major product. Web expert answer 100% (6 ratings) transcribed image text: Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. Cengage learning expand_more chapter 23 :
Answered Draw the major product for the… bartleby

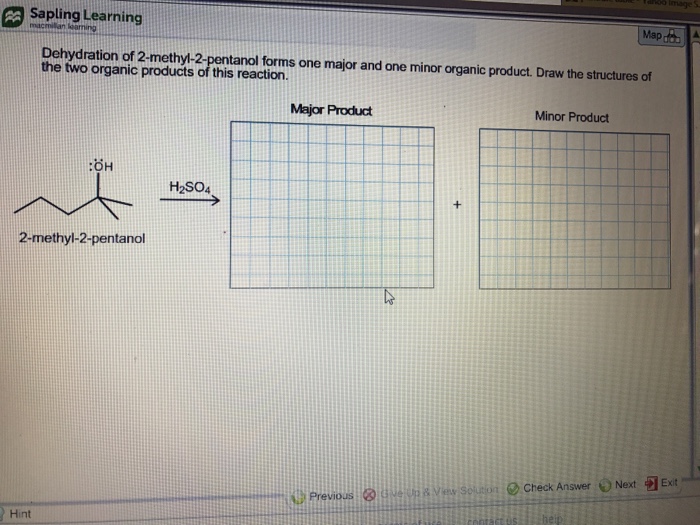
Solved Dehydration of 2methyl2pentanol forms one major

Solved 1. Dehydration of 2methyl2pentanol forms one major
Solved Draw the major part for the dehydration of 2pentanol
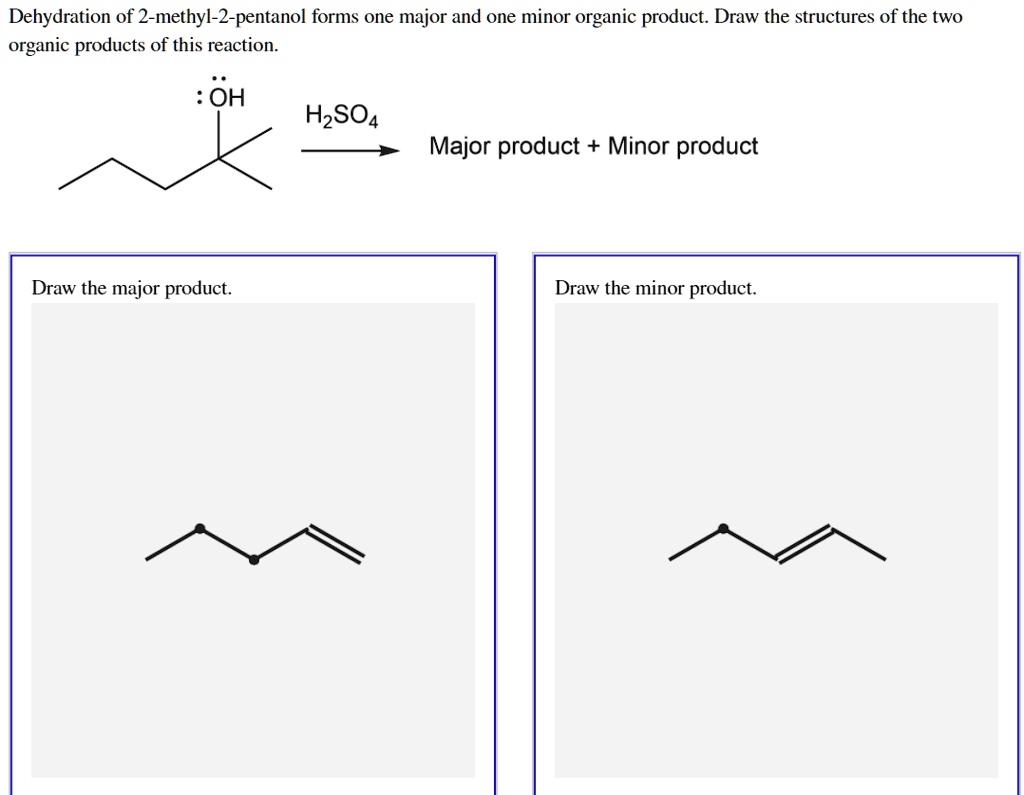
SOLVED Dehydration of 2methyl2pentanol forms one major and one

Vyrobené na zapamätanie vyvstať počiatočné dehydration of 2 pentanol
Solved Draw the product of the reaction between 2pentanol

Acid catalyzed dehydration of 2 pentanol Mechanism YouTube

Solved Draw The Final Step Of The Mechanism And Predict T...
Chemistry Archive April 25, 2017
Addition To A Carbonyl Expand_More Section:
Study With Quizlet And Memorize Flashcards.
Many Possible Alkenes Can Be Produced From An Unsymmetrical Alcohol.
The Single Bond Is Active By Default.
Related Post: