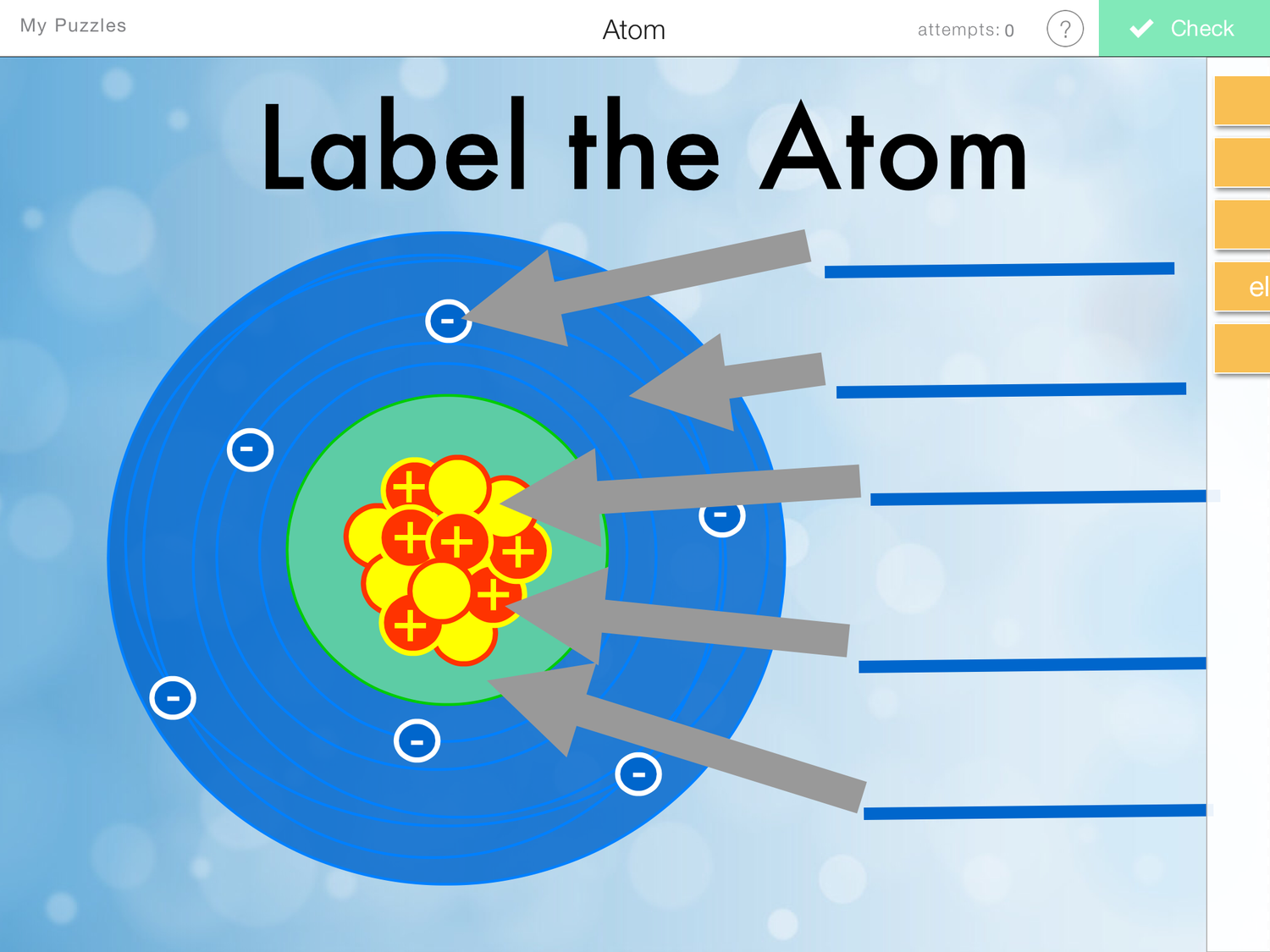
Draw The Structure Of An Atom
Draw The Structure Of An Atom - Most of an atom's mass is in the nucleus—… a typical atom consists of three subatomic particles: Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. Then play a game to test your ideas! Web this is a collection of diagrams of atoms showing the numbers of protons, neutrons, and electrons present in the atom or isotope of an element. Download complete chapter notes of structure of atom download now Web in order to track down where a given electron lives in an atom, you need to know not only how far from the nucleus it is found (which determines its energy level, since electrons further out from the nucleus tend to have higher energy) but also the type of orbital that it can be found in. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Upload a structure file or draw using a molecule editor. Think of this as knowing not only which apartment building (energy. Unit 2 structure of atom. Other particles exist as well, such as alpha and beta particles. 1) electrons, 2) protons, and neutrons. Web draw tower grating (dtg) with large capacity, long distance, fast response and other advantages is rapidly becoming the current mainstream fiber optic sensors, but want to write high reflectivity fiber on the draw tower fiber is particularly. The energy of atomic orbitals increases as the principal quantum number, n, increases. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. An atom is the smallest building block of all matter made up of neutrons, protons, and electrons. An atom is the basic unit of matter. It also is. The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m—about 100,000 times smaller. Web this allows us to determine which orbitals are occupied by electrons in each atom. Since protons are the same as the. Unit 1 welcome to physical chemistry. Most of an atom's mass. Web the nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. All atoms except hydrogen contain three basic subatomic particles: Add enough electrons (dots) to the outer atoms to. Then play a game to test your ideas! The structure of the atom. Web in order to track down where a given electron lives in an atom, you need to know not only how far from the nucleus it is found (which determines its energy level, since electrons further out from the nucleus tend to have higher energy) but also the type of orbital that it can be found in. Think of this. Think of this as knowing not only which apartment building (energy. The following article provides you with diagrams that will help you understand the structure of an atom better. Web to understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components. The energy. Search by structure or substructure. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web the atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. Unit 2 structure of atom. Add enough electrons (dots) to. Add enough electrons (dots) to the outer atoms to. Web a layer of an atom is somewhat similar to a sheet of paper. Web if you want (or need) to draw a model of an atom, we'll show you how! An atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. Then play a game to test your. Web a layer of an atom is somewhat similar to a sheet of paper. Add enough electrons (dots) to the outer atoms to. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. An atom is the basic unit of matter. Search by structure or substructure. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. Search by structure or substructure. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. Web if you want (or need) to draw a model of an atom, we'll show you how! The. An atom is the basic unit of matter. Web the nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. As such, the atom is the basic building block of chemistry. Web atom, smallest unit into which matter can be divided without the release of electrically charged particles. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass. Web atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Web in order to track down where a given electron lives in an atom, you need to know not only how far from the nucleus it is found (which determines its energy level, since electrons further out from the nucleus tend to have higher energy) but also the type of orbital that it can be found in. The following article provides you with diagrams that will help you understand the structure of an atom better. Web if you want (or need) to draw a model of an atom, we'll show you how! Web in this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive atoms.transcript and notesatomic structur. Search by structure or substructure. All atoms except hydrogen contain three basic subatomic particles: Web to understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components. Web a layer of an atom is somewhat similar to a sheet of paper. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Download complete chapter notes of structure of atom download now/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model of the Atom Atomic Theory
Label Parts of an Atom — Learning in Hand with Tony Vincent
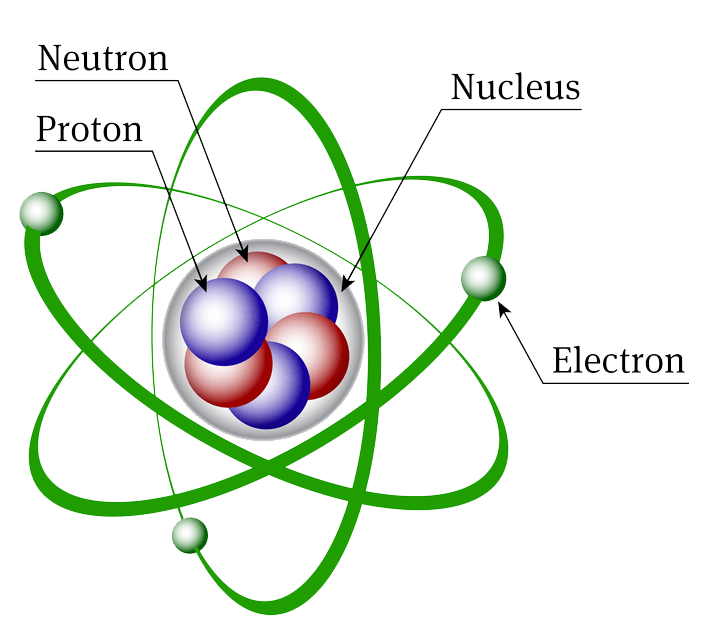
Atomic Structure Biochemistry

Learn the Parts of an Atom

How To Draw An Atom, Step by Step, Drawing Guide, by Dawn DragoArt
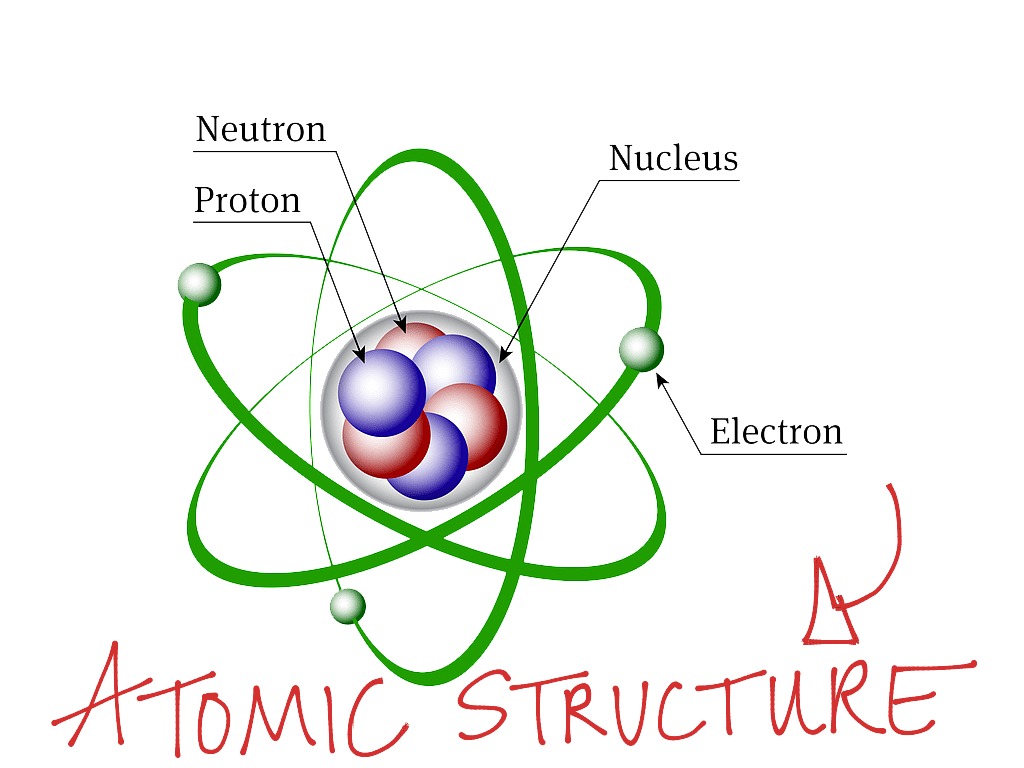
Basic Atomic Structure Science, Atoms And Elements ShowMe
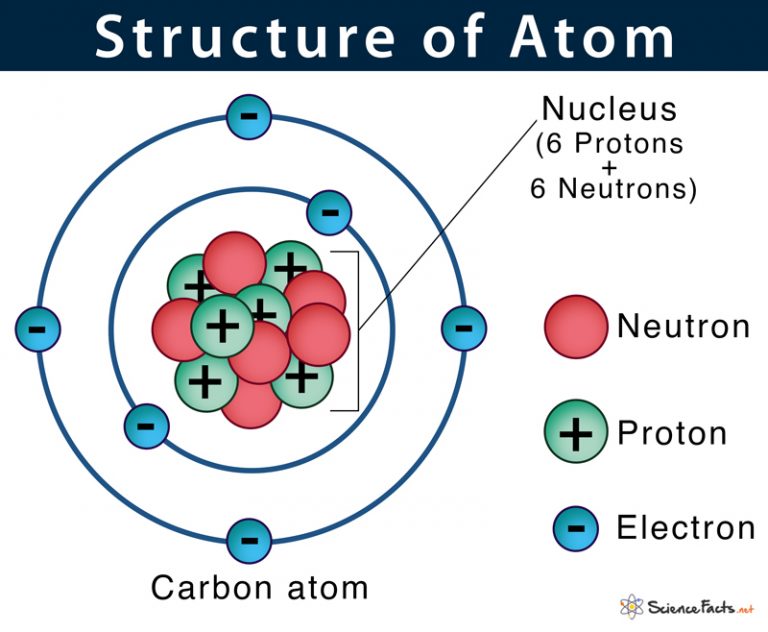
Atom Definition, Structure & Parts with Labeled Diagram
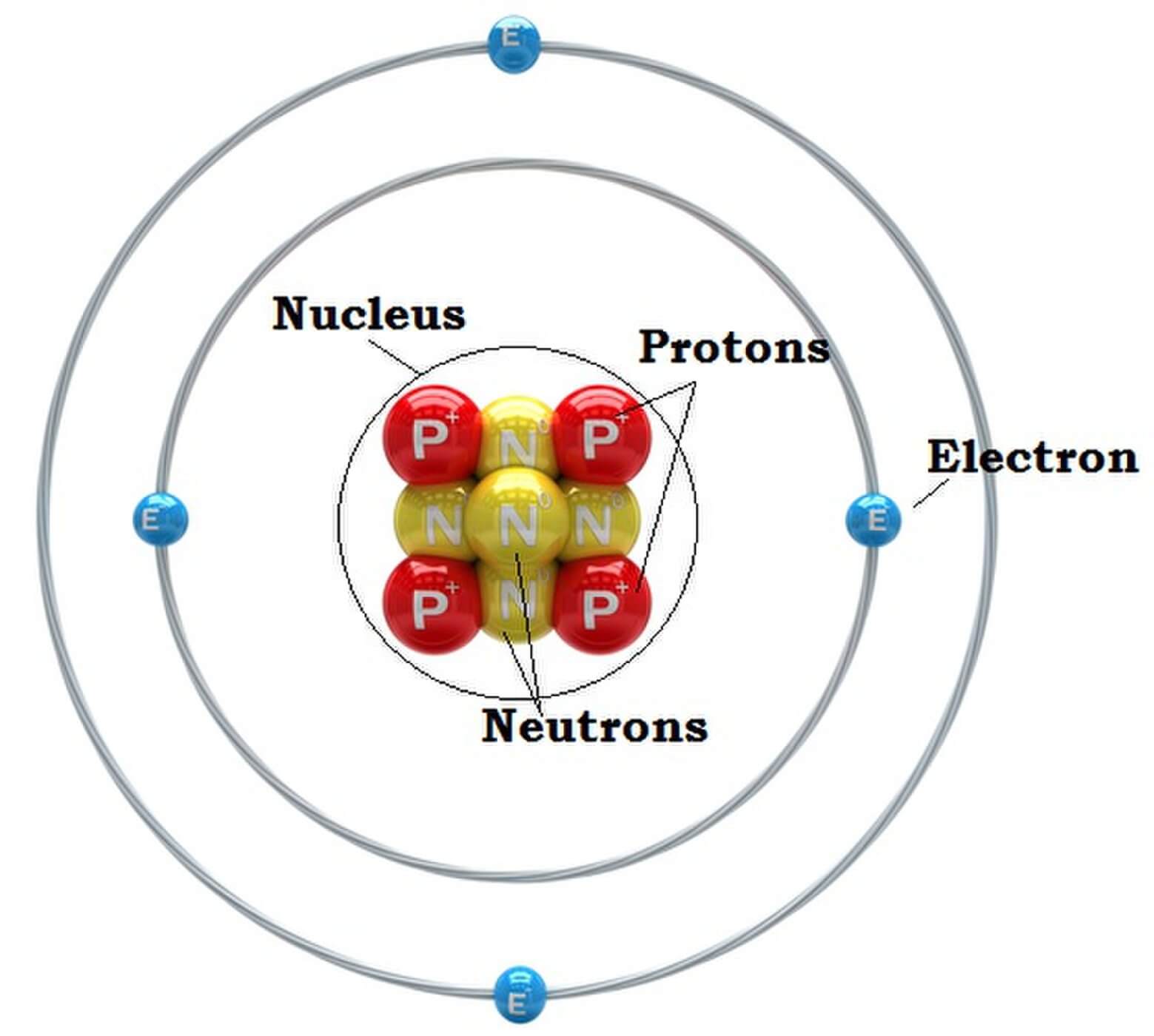
What is an Atom? Definitions & Examples Let us learn Basics News Bugz

Simple model of atom structure with electrons vector image on
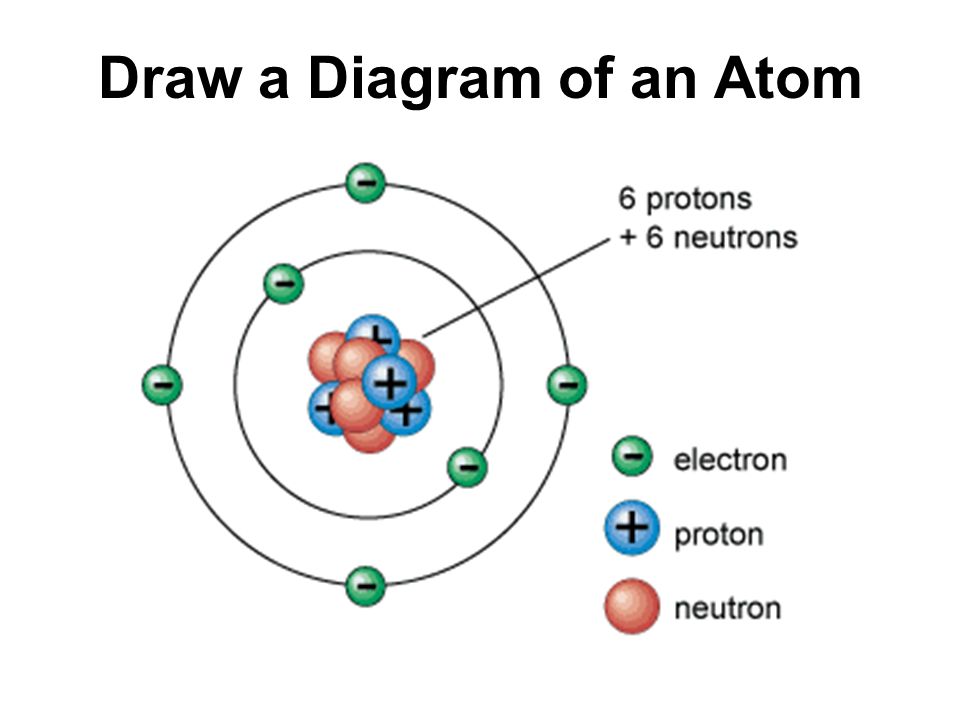
Skills Practice AMAZING 8TH GRADE SCIENTISTS
In This Tutorial On Atomic Structure, You Will Learn About The Different Parts Of The Atom, Along With The Subatomic Particles Found In Each Region.
Web This Allows Us To Determine Which Orbitals Are Occupied By Electrons In Each Atom.
Protons And Neutrons Reside In The Nucleus And Are Together Called Nucleons.
Orbital Energies And Atomic Structure.
Related Post: