Drawing Of A Hydrogen Atom
Drawing Of A Hydrogen Atom - Questions tips & thanks want to join the conversation? Web this web page shows the scale of a hydrogen atom. Web 5.4k views 7 years ago. Check how the prediction of the model matches the experimental results. And so you see it forms this tetrahedral shape, it's pretty close to a tetrahedron. Web solving schrödinger’s equation for hydrogen. Historically, bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen. A skeletal representation ignores the hydrogen atoms and their bonds to carbon. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Identify the physical significance of each of the quantum numbers (n, l, m) of the hydrogen atom; U(r, θ, ϕ) = − ke2 r. Web i'll draw it as a little green circle there. A skeletal representation ignores the hydrogen atoms and their bonds to carbon. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Check how the prediction of the model matches the. Web proposed in 1904 by j. Web hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Web summarize how bohr’s quantum model of the hydrogen atom explains the radiation spectrum of atomic hydrogen historically, bohr’s model of the hydrogen atom is the very first model of atomic structure that correctly explained. The. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). You have the covalent bond to one hydrogen atom right over there. Web proposed in 1904 by j. Web following hydrogen is the noble. Web i'll draw it as a little green circle there. Web 5.4k views 7 years ago. Web summarize how bohr’s quantum model of the hydrogen atom explains the radiation spectrum of atomic hydrogen. And so you see it forms this tetrahedral shape, it's pretty close to a tetrahedron. You have the covalent bond to one hydrogen atom right over there. The second electron also goes into the 1s orbital and fills that orbital. You have the covalent bond to one hydrogen atom right over there. The helium atom contains two protons and two electrons. Atom labels for all other elements are shown. Distinguish between the bohr and schrödinger models of the atom The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the coulomb force. Another lone pair of electrons back here. Web a hydrogen atom is an atom of the chemical element hydrogen. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1,. The helium atom contains two protons and two electrons. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. It also misses out all the carbon atoms. The first electron has the same four quantum numbers as the hydrogen atom. Web in this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Distinguish between the bohr and schrödinger models of the atom Web the electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. The helium atom contains two protons and two electrons.. Web following hydrogen is the noble gas helium, which has an atomic number of 2. The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. Also, helium is shown in group 8a, but it only has two valence electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1,. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Web this web page shows the scale of a hydrogen atom. It also misses out all the carbon atoms. Atom labels for all other elements are shown. Also, helium is shown in group 8a, but it only has two valence electrons. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. Questions tips & thanks want to join the conversation? The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). Identify the physical significance of each of the quantum numbers (n, l, m) of the hydrogen atom; For hydrogen, the potential energy function is simply: Web in this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout. Web for the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 å (52.9 pm), which is exactly the radius calculated by bohr for the n = 1 orbit. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Web solving schrödinger’s equation for hydrogen. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. U(r, θ, ϕ) = − ke2 r. Web this web page shows the scale of a hydrogen atom. The second electron also goes into the 1s orbital and fills that orbital. A skeletal representation ignores the hydrogen atoms and their bonds to carbon. Atom labels for all other elements are shown.
Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy

Diagram Representation Of The Element Hydrogen Stock Vector Image

Bohr Atomic Model Of Hydrogen

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
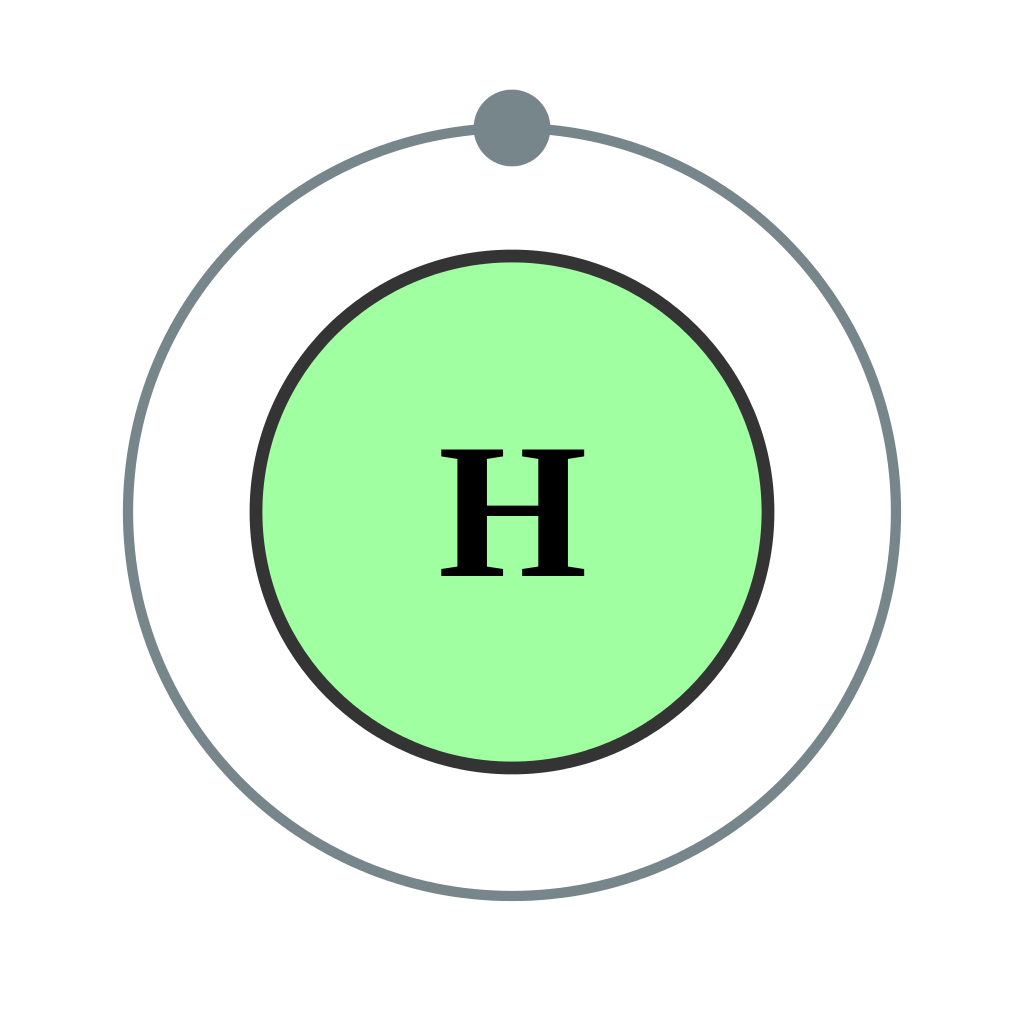
FileElectron shell 001 Hydrogen no label.svg Wikipedia

Describe Bohr’s model of the hydrogen atom. bitWise Academy

Hydrogen atom on white background Royalty Free Vector Image
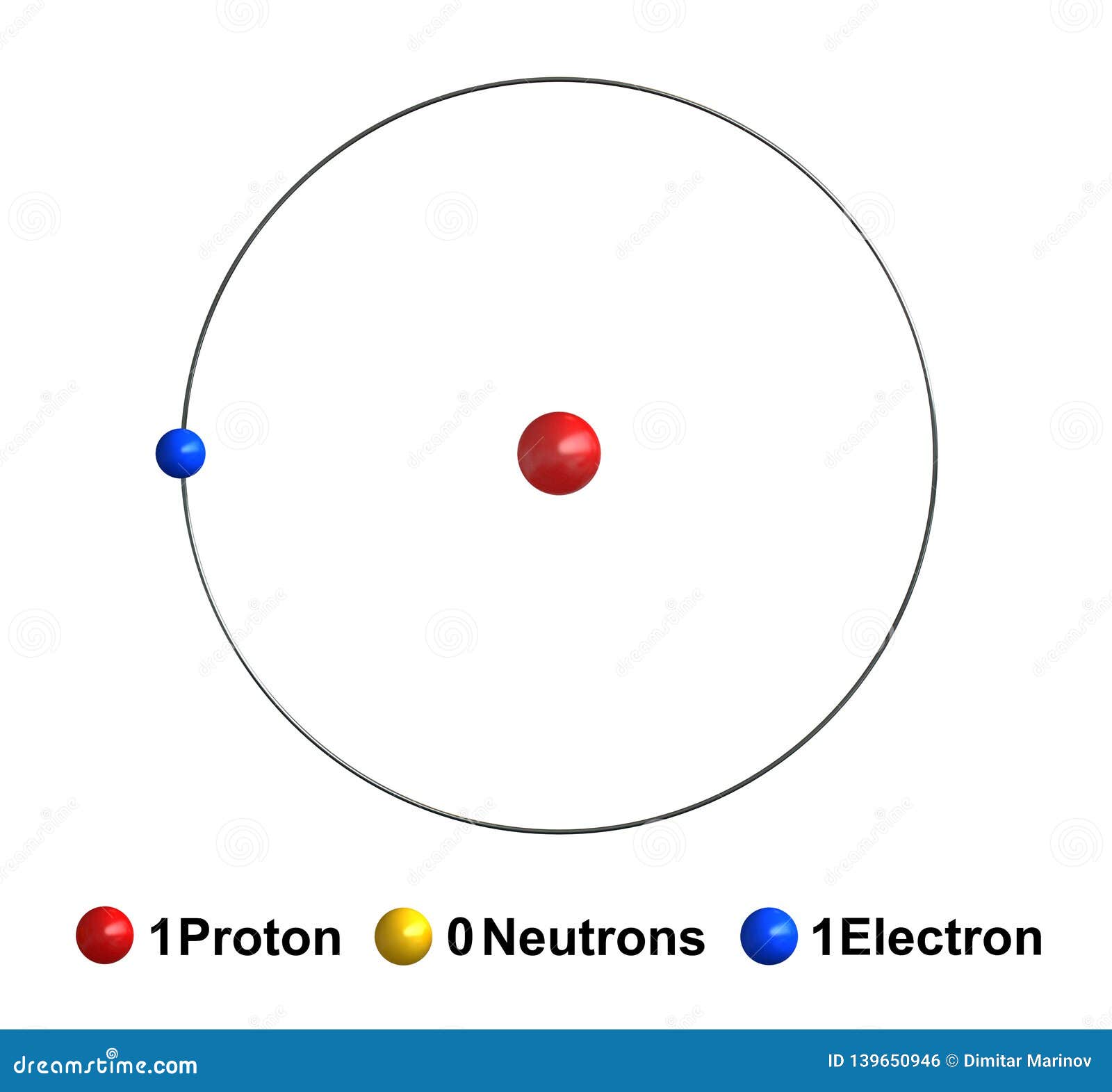
Hydrogen stock illustration. Illustration of education 139650946

Hydrogen Definition, Structure, Properties, and Uses

Diagram representation element hydrogen Royalty Free Vector
We’ll Use A Bohr Diagram To Visually Represent Where The Electrons Are Around The Nucleus Of The H Atom.
And Then You Have The Covalent Bond To The Other Hydrogen Atom.
Web I'll Draw It As A Little Green Circle There.
Electron Shells Consist Of One Or More Subshells, And Subshells Consist Of One Or More Atomic Orbitals.
Related Post: