Drawing Of Nitrogen
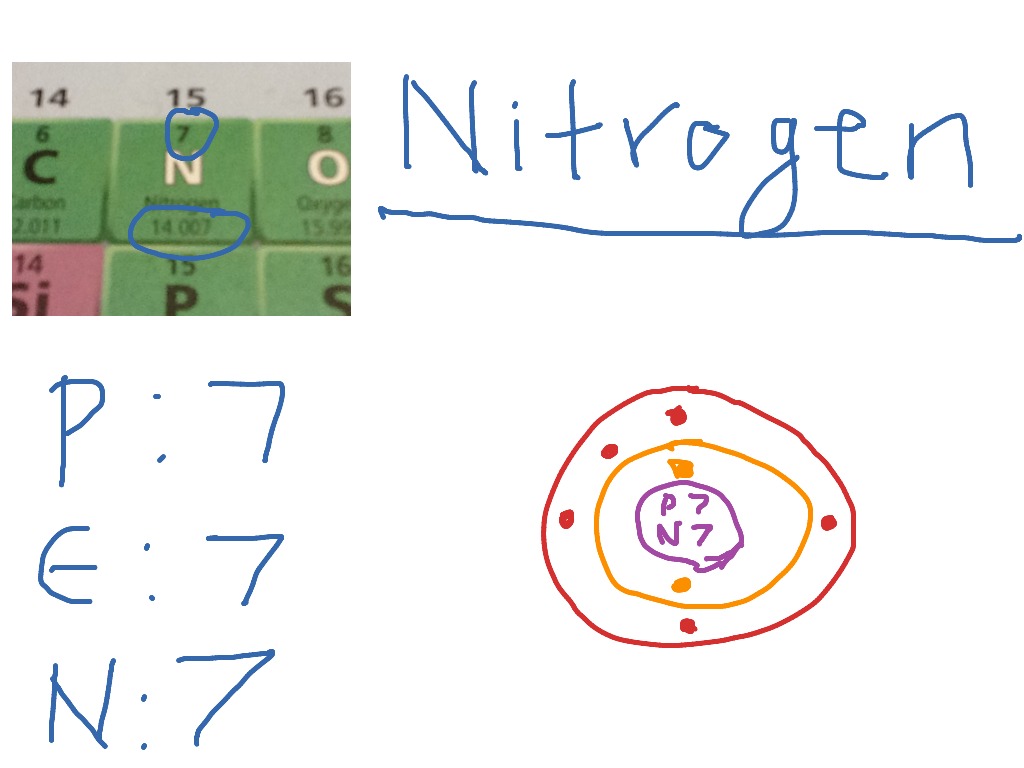
Drawing Of Nitrogen - Each oxygen atom has two bonds. I show you where nitrogen is on the periodic table and how to determine how many. Nitrogen exists in the atmosphere as n 2 gas. Figure 2 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. How many electrons would nitrogen lewis dot structure have? Nitrogen has 2 electrons in its first shell and 5 in its second. Remember, we kept these separate from the p set as a simplification. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Below is the electron dot structure for a nitrogen molecule: As per the molecule n2, it has two atoms of nitrogen. Nitrate is. Below is the electron dot structure for a nitrogen molecule: Nitrogen exists in the atmosphere as n 2 gas. The diatomic nitrogen molecule elements come as members of the nitrogen family group from the periodic table. Every time that you see nitrogen with three bonds, let me draw these in here, one, two, three. Two each go into the s. Web in this video we'll look at the atomic structure and bohr model for the nitrogen atom (n). In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. When animals eat the plants, they acquire. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. Each form of nitrogen has different characteristics that affect how it reacts in the substrate and how plants uptake and assimilate it. There are a total of ten electrons. Web draw a lewis electron dot diagram for an atom or a monatomic. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Nitrogen exists in the atmosphere as n 2 gas. How many electrons would nitrogen lewis dot structure have? We’ll use a bohr diagram to visually represent where the electrons. Web steps to draw the lewis structure of n2. So three bonds and one lone pair of electrons, the formal charge is equal to zero. 2,5, it has five electrons in its outermost valence shell. The shell closest to the nucleus is. Find the total valence electrons for the n2 molecule. Each hydrogen atom has one bond. Two each go into the s s bonding and s s * antibonding levels. As per the molecule n2, it has two atoms of nitrogen. Web we found the nitrogen to have a formal charge of zero. It is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. Each oxygen atom has two bonds. Put the least electronegative atom in the center. Below is the electron dot structure for a nitrogen molecule: Web each nitrogen has five valence electrons. Nitrogen exists in the atmosphere as n 2 gas. Web we found the nitrogen to have a formal charge of zero. Here in this post, we described a step by step method to construct the n2 lewis structure. Nitrogen has 2 electrons in its first shell and 5 in its second. Each nitrogen atom has three bonds. Each hydrogen atom has one bond. 2,5, it has five electrons in its outermost valence shell. Each carbon atom has four bonds. Figure 2 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Find the total valence electrons for the n2 molecule. Put the least electronegative atom in the center. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web nitrogen is cycled naturally by living organisms through the ‘nitrogen cycle’. Remember, we kept these separate from the p set as a simplification. Pharmacy biology science structure business success Nitrogen exists in the atmosphere as n 2 gas. The shell closest to the nucleus is. Nitrate is the most mobile form of nitrogen and easily dissolves in water. How many electrons would nitrogen lewis dot structure have? Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. There are a total of ten electrons. Two each go into the s. So we have a pattern. Below is the electron dot structure for a nitrogen molecule: It is a visual representation of the electron configuration of nitrogen, which helps us understand its chemical bonding and molecular structure. Here in this post, we described a step by step method to construct the n2 lewis structure. Web each nitrogen has five valence electrons. In the periodic table, nitrogen is placed in group 5 across period 2.
Nitrogen Wikipedia
How to draw a Nitrogen Atom Science ShowMe

Steps of Nitrogen Cycle There are 5 steps of nitrogen cycle that help

3d render of atom structure of nitrogen isolated over white background
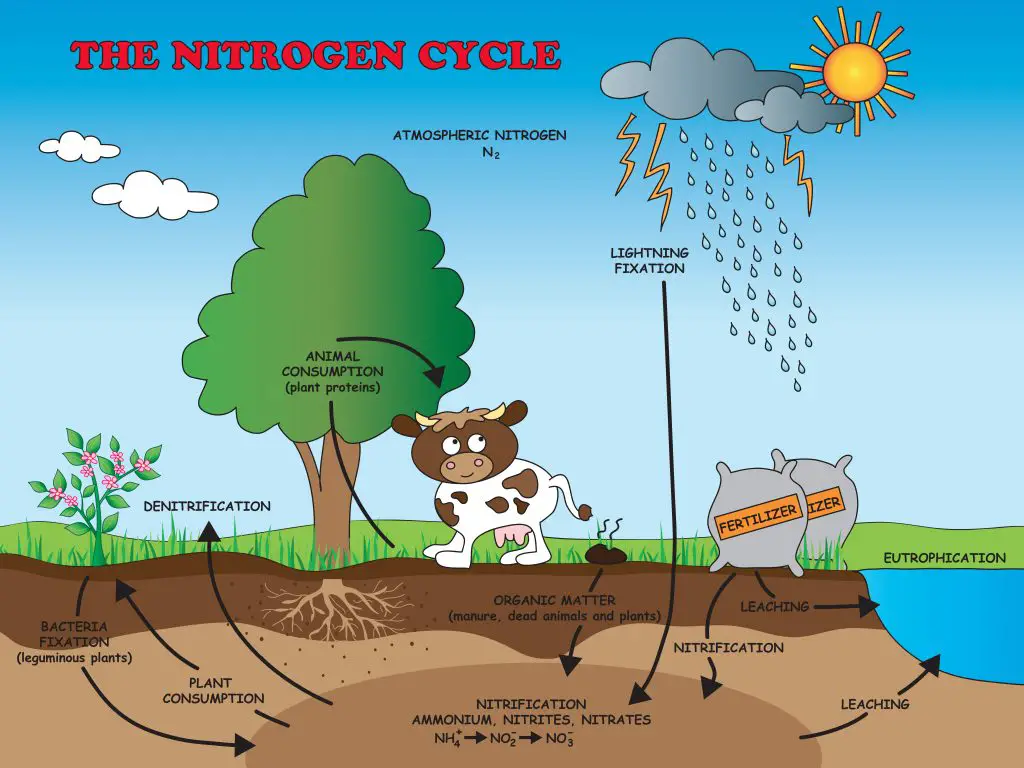
Nitrogen Cycle Facts for Kids (Explained!) Education site

Understanding the Nitrogen Cycle Beginners Education AlgaeBarn
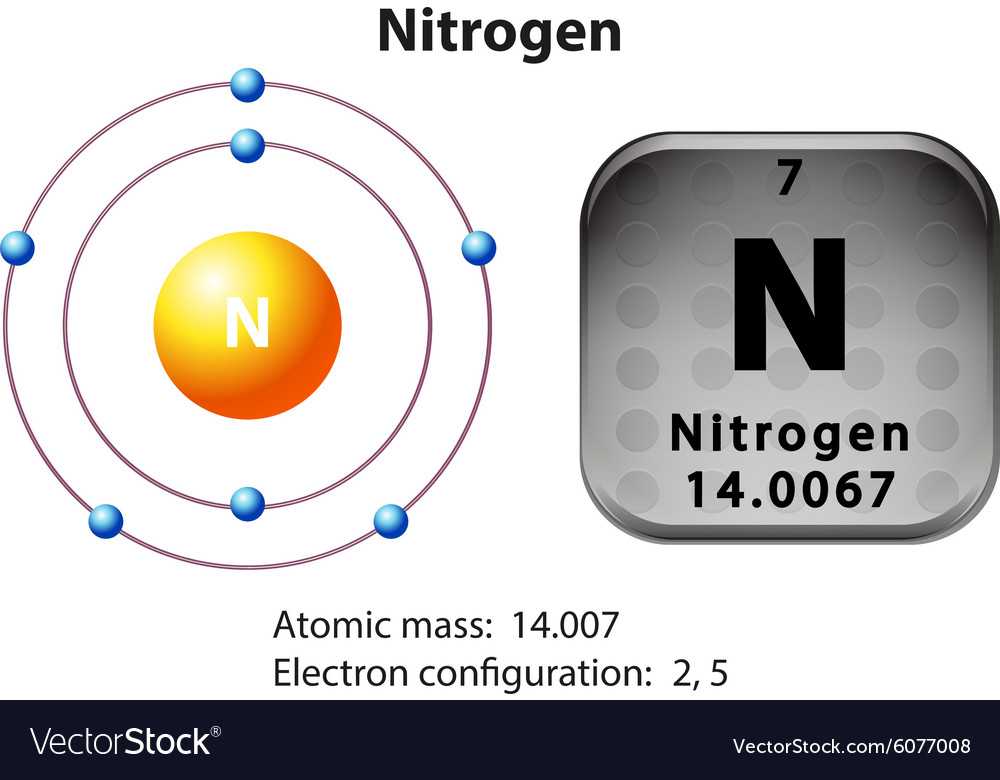
Symbol and electron diagram for nitrogen Vector Image

Nitrogen Facts, Symbol, Discovery, Properties, Uses
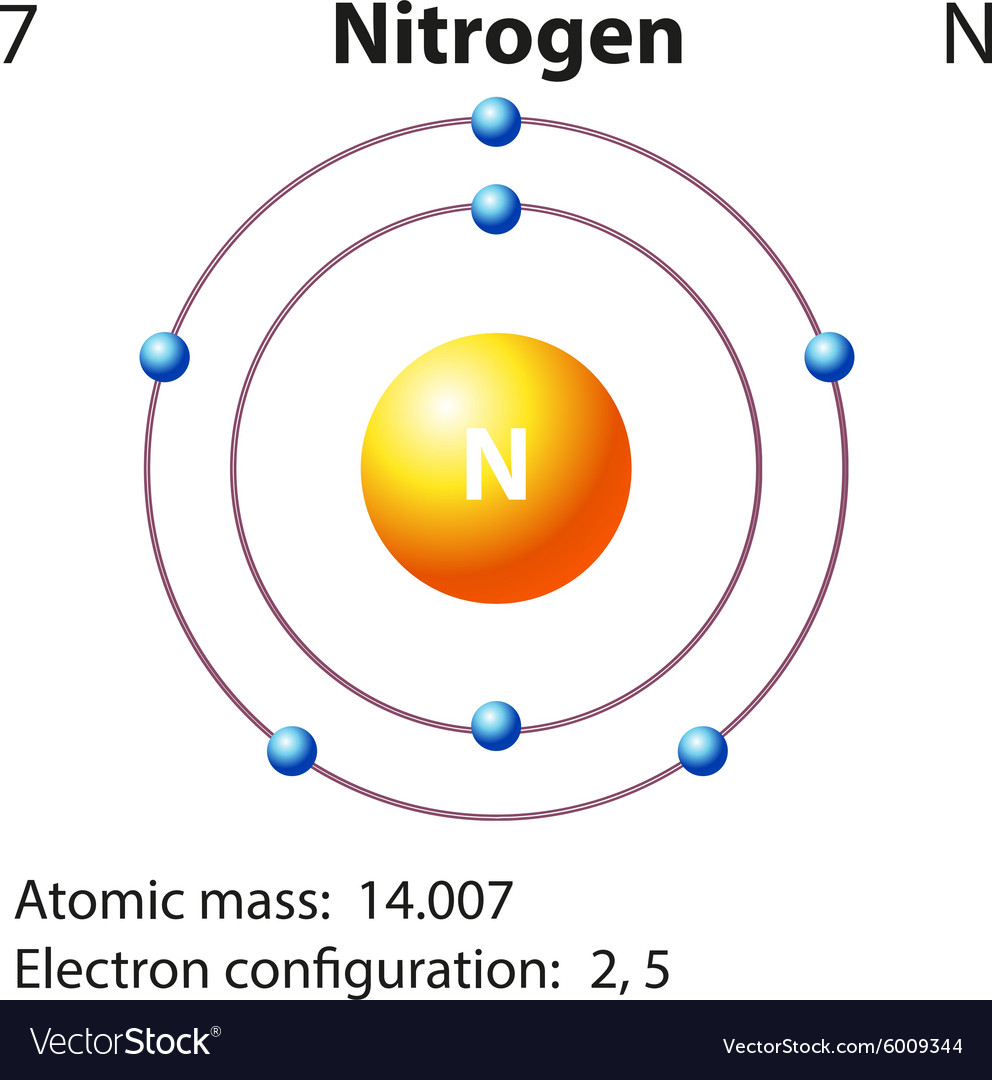
Diagram representation element nitrogen Royalty Free Vector
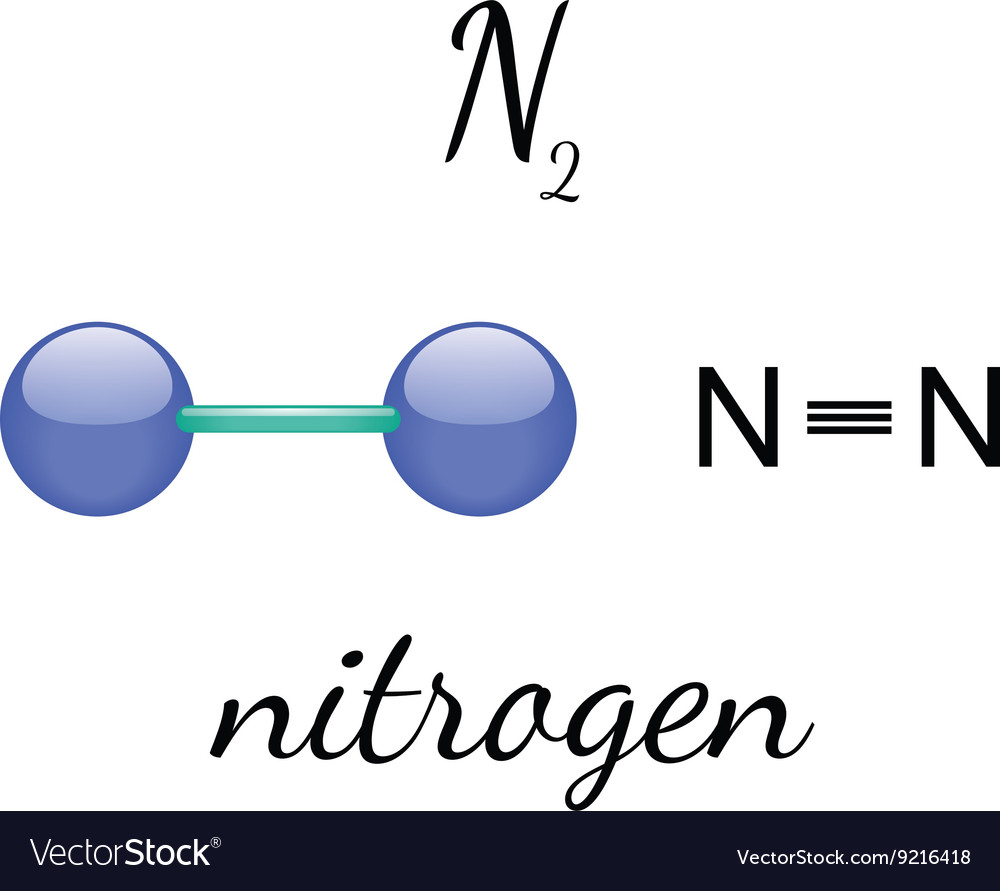
N2 nitrogen molecule Royalty Free Vector Image
Each Hydrogen Atom Has One Bond.
Find The Total Valence Electrons For The N2 Molecule.
Each Nitrogen Atom Has Three Bonds.
Web Bohr Diagrams Show Electrons Orbiting The Nucleus Of An Atom Somewhat Like Planets Orbit Around The Sun.
Related Post: