Element Radius Chart
Element Radius Chart - Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Click on radio button atomic radius. 1 å = 1 × 10−10 m = 100 pm. Atoms consist of a nucleus with positively charged protons and neutral neutrons surrounded by shells of electrons. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is defined as half the distance between the nuclei of two adjacent atoms in a metallic element. Atomic radii can be obtained from quantum mechanical calculations or. Slater are an empirical set of atomic radii derived by the careful comparison of bond lengths in over 1200 bond types in ionic, metallic, and covalent crystals and molecules (reference 1). Web the periodic table of the elements (including atomic radius) element name. This is due to the way electrons form shells around the nucleus. These values derived by j.c. Going across a period, the main group elements tend to decrease in atomic radius due to the increased nuclear charge. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. This special periodic table shows the relative size of atoms. 1 å = 1 × 10−10 m = 100 pm. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Web atomic radii (clementi) gallery of images. Going across a period,. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Click on radio button atomic radius.. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. Slater are an empirical set of atomic radii derived by the careful comparison of bond lengths in over 1200 bond types in ionic, metallic, and covalent crystals and molecules (reference 1). Web the atomic radius of a chemical element is the distance from the. How many electrons are filled out in each subshell? The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear. Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Web interactive. The relative size of the atoms follows a set of trends on the periodic table. Atomic radius is the measure of the distance from the centre of the nucleus to the outer electron. This atomic radius (calculated) table gives the atomic radius (calculated) of all the elements of periodic table in pm. (a) the covalent atomic radius, rcov, is half. Atoms consist of a nucleus with positively charged protons and neutral neutrons surrounded by shells of electrons. Web atomic radii (clementi) gallery of images. Definitions of the atomic radius. The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear. Visualize trends, 3d orbitals, isotopes,. Web the periodic table contains nist’s latest critically evaluated data for atomic properties of the elements. (a) the covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Many references give table of atomic radii. Sometimes in text books and other sources,. Web the atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Web atomic radius of all the elements are mentioned in the chart below. This is due to the way electrons form shells around the nucleus. Atomic. If the two atoms are of the same kind, then the covalent radius is simply one half of the bond length. Explore the chemical elements through this periodic table. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is. Web the periodic table of the elements by webelements. If the two atoms are of the same kind, then the covalent radius is simply one half of the bond length. Definitions of the atomic radius. Web the covalent atomic radius (r cov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius (r met) is defined as half the distance between the nuclei of two adjacent atoms in a metallic element. Web the atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Atomic radii can be obtained from quantum mechanical calculations or. Below mentioned radii are the van der waals radius in picometer (pm)). Click on 'element atomic number', 'element symbol', 'element name' and 'element atomic radius (calculated) ' headers to sort. The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear. Going across a period, the main group elements tend to decrease in atomic radius due to the increased nuclear charge. Web atomic radius of all the elements are mentioned in the chart below. Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. When clicking the radio button (3) the atomic radius of all the elements will be viewed simultaniously in the text fields located above the symbols. Atoms consist of a nucleus with positively charged protons and neutral neutrons surrounded by shells of electrons. Atomic radius is the measure of the distance from the centre of the nucleus to the outer electron..png)
Periodic Table Of The Elements Atomic Radius vrogue.co

Atomic Radius of Elements The Periodic Table
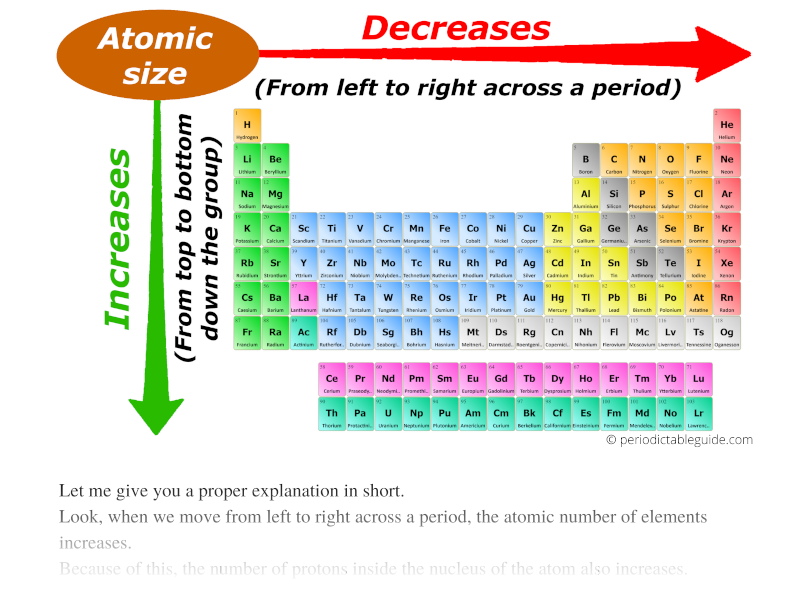
Periodic Table Showing Atomic Radius

This periodic table chart shows the relative sizes of each element

Atomic Radius and Ionic Radius

Atomic Radius of Elements
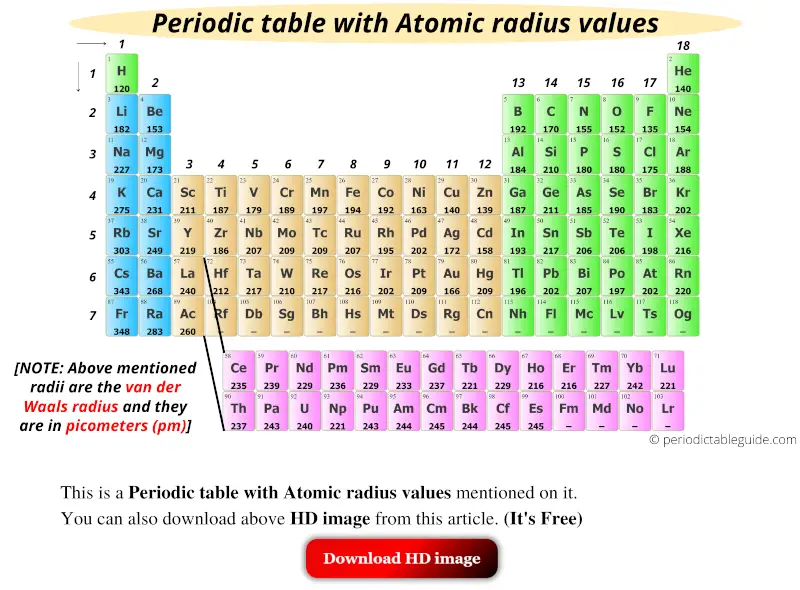
Get the Periodic table with Atomic radius values (Img+Chart)
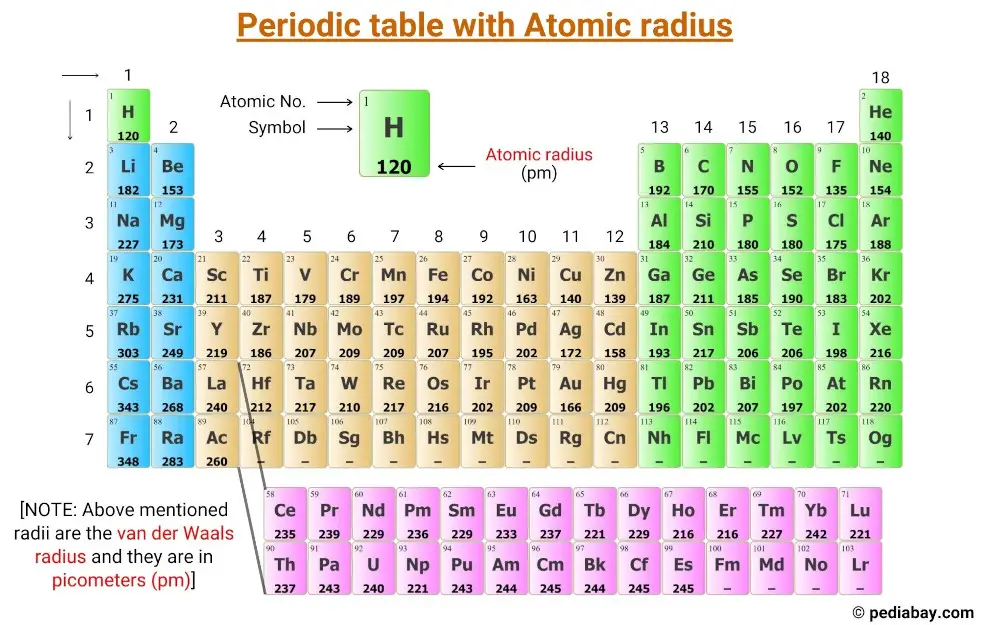
Atomic Radius of Elements (With Periodic table Chart) Pediabay

Elements, Atomic Radii and the Periodic Radii

Atomic Sizes and Radii Charts for Chemistry
Web The Simplest Answer Is That Potassium Has Higher Valence Energy Level (Energy Level 4) Than Lithium (Energy Level 2), Which Has Greater Distance From The Nuclear Thus Has Bigger Radius.
How Many Electrons Are Filled Out In Each Subshell?
Web Click On Periodic Table Push Button.
Elements In The Periodic Table Are Organized Into Periods And Groups.
Related Post: