Ethylene Glycol Freezing Point Chart
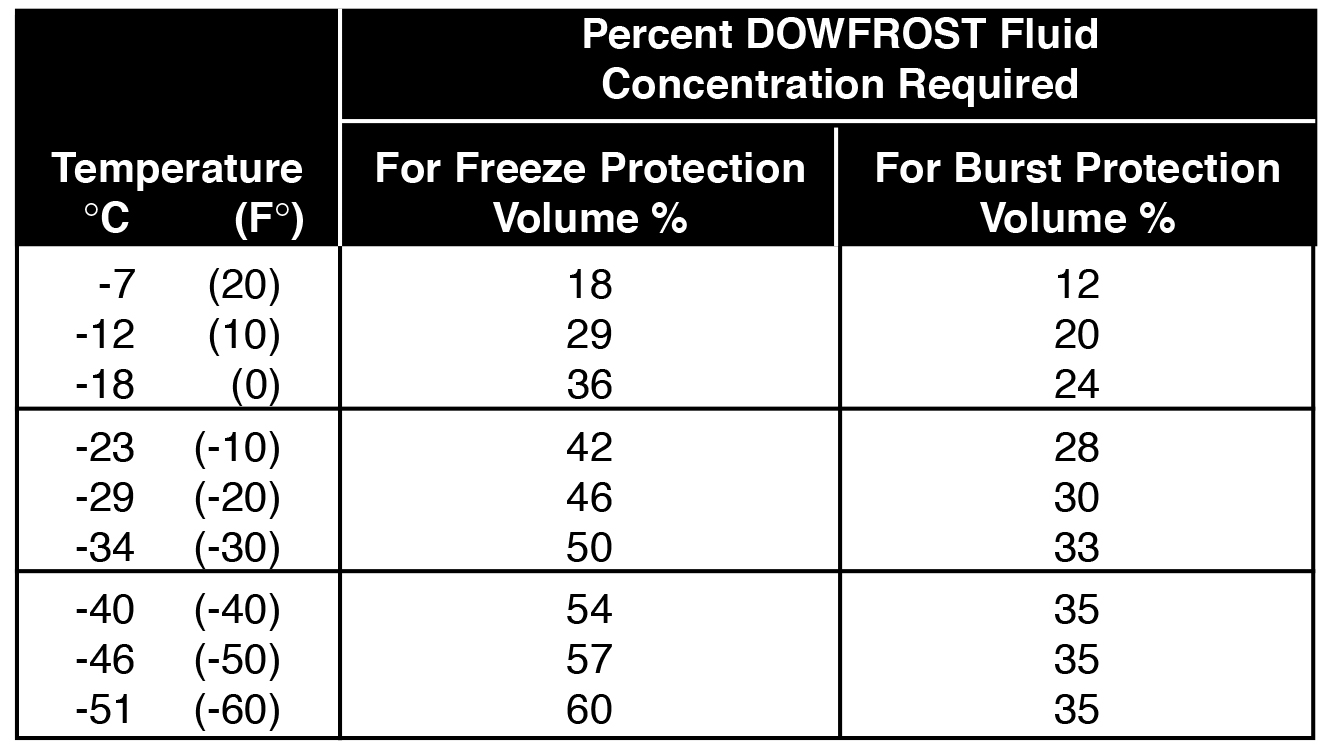
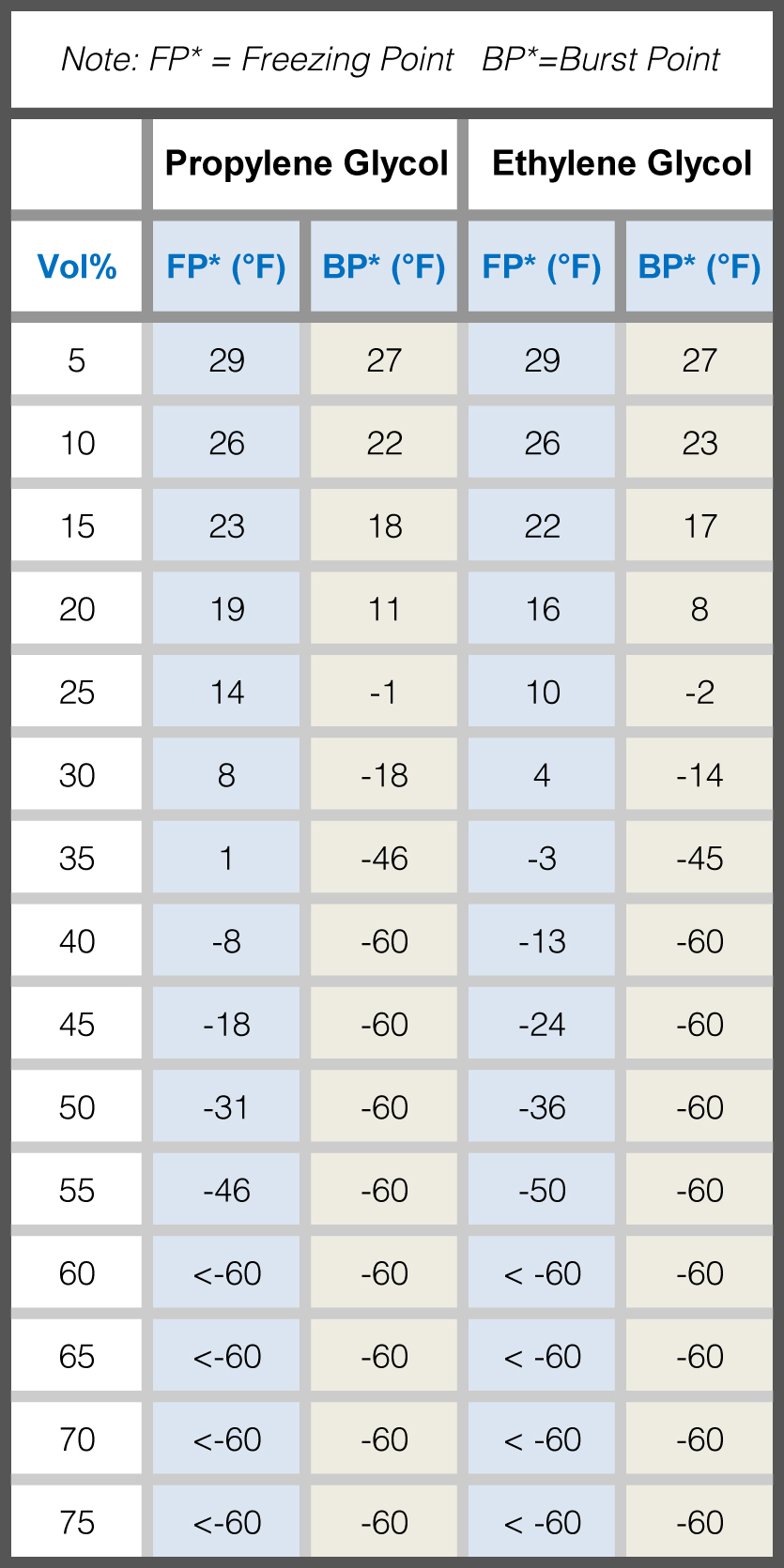
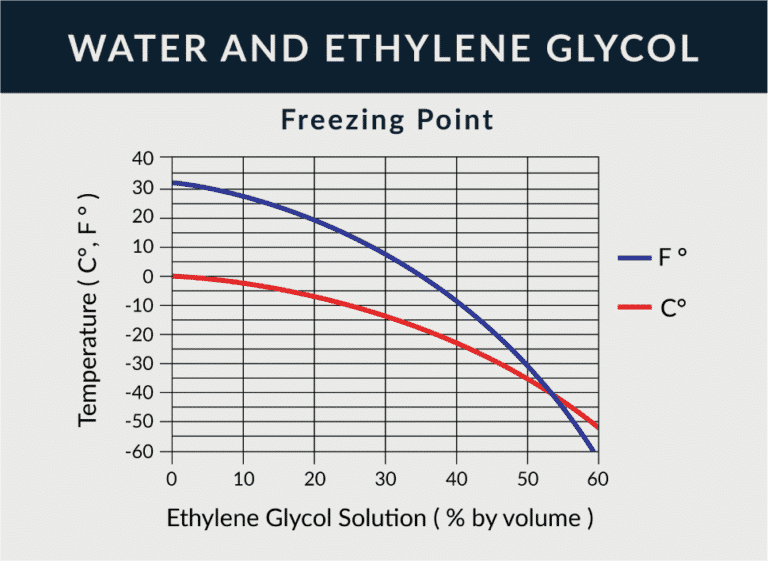
Ethylene Glycol Freezing Point Chart - Specific gravity is referenced to water at 15.6 °c. Web in example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of ethylene glycol at 100°c is 85.1 mmhg less than the vapor pressure of pure water. Web after factoring for temperature correction, use the appropriate freeze point chart on the next page to match the specific gravity of the sample to the freezing point of the water. 40% mokon concentration by volume. See table 2 for dynalene ethylene glycol freezing points for various concentrations. Web for effective freeze protection of water systems, a chart is listed below to provide the recommended inhibited propylene glycol concentrations for dowfrost™ and dowfrost™ hd glycol. Time to freeze piping formulas and calculator. Web ethylene glycol as an antifreeze is based on its ability to lower the freezing point when mixed with water. At that same temperature, the system would need a concentration of about 30% dowfrost hd propylene glycol for the freeze protection and about 21%. This is very important for closed loop systems that may be exposed to freezing conditions. See table 2 for dynalene ethylene glycol freezing points for various concentrations. Web ethylene glycol \(\left( \ce{c_2h_6o_2} \right)\) is a molecular compound that is used in many commercial antifreezes. Identify glycol to water ratio. For cold systems, you can use ~2% less eg than pg to achieve the same freeze protection. 40% mokon concentration by volume. This is very important for closed loop systems that may be exposed to freezing conditions. Table obtained from lange's handbook of chemistry, 10th ed. Web pure ethylene glycol freezes at about −12 °c (10.4 °f) but, when mixed with water, the mixture freezes at a lower temperature. The end uses for ethylene glycol are numerous (see table 1). Specific gravity. Time to freeze piping formulas and calculator. At that same temperature, the system would need a concentration of about 30% dowfrost hd propylene glycol for the freeze protection and about 21%. Table obtained from lange's handbook of chemistry, 10th ed. For example, a mixture of 60% ethylene glycol and 40% water freezes at −45 °c (−49 °f). We stated (without. Web view glycol concentration chart or use our calculator to determine freezing point and burst point of propylene glycol and ethylene glycol. Web in example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of ethylene glycol at 100°c is 85.1 mmhg less than the vapor pressure of pure water. For cold systems, you can use ~2%. Time to freeze piping formulas and calculator. † typical properties, not to be construed as specifications. Web freezing point of aqueous solutions. 40% mokon concentration by volume. For example, a mixture of 60% ethylene glycol and 40% water freezes at −45 °c (−49 °f). Water boiling temperature vs pressure in vacuum table chart. Web freezing point of aqueous solutions. Web recommends a glycol solution that can maintain a freezing point of at least 10°f below the lowest anticipated temperature. † typical properties, not to be construed as specifications. At that same temperature, the system would need a concentration of about 30% dowfrost hd propylene. Focuses on ethylene glycol solutions having a specific freezing point temperatures. For example, a mixture of 60% ethylene glycol and 40% water freezes at −45 °c (−49 °f). Web in example 13.8.1, we calculated that the vapor pressure of a 30.2% aqueous solution of ethylene glycol at 100°c is 85.1 mmhg less than the vapor pressure of pure water. The. Specific gravity is referenced to water at 15.6 °c. Time to freeze piping formulas and calculator. Temperature corrections for specific gravity hydrometers (15.5ºc/60ºf) We stated (without offering proof) that this should result in a higher boiling point for the solution compared with pure water. This is very important for closed loop systems that may be exposed to freezing conditions. At that same temperature, the system would need a concentration of about 30% dowfrost hd propylene glycol for the freeze protection and about 21%. Web boiling point (°c) k b (°c⋅kg/mol) freezing point (°c) k f (°c⋅kg/mol) data source; Thermophysical properties are measured in low temperature range. We stated (without offering proof) that this should result in a higher boiling. Web ethylene glycol \(\left( \ce{c_2h_6o_2} \right)\) is a molecular compound that is used in many commercial antifreezes. For example, a mixture of 60% ethylene glycol and 40% water freezes at −45 °c (−49 °f). † typical properties, not to be construed as specifications. Water boiling graph curve at 1 atmosphere. Specific gravity is referenced to water at 15.6 °c. This is very important for closed loop systems that may be exposed to freezing conditions. Calculate the freezing point of a solution of \(400 \: Specific gravity is referenced to water at 15.6 °c. Web a ethylene glycol concentrations greater than 92% are not attainable with dowtherm 4000 fluid. Web pure ethylene glycol freezes at about −12 °c (10.4 °f) but, when mixed with water, the mixture freezes at a lower temperature. Boiling point of water at various pressures. At that same temperature, the system would need a concentration of about 30% dowfrost hd propylene glycol for the freeze protection and about 21%. Archived from the original (pdf) on 27 september 2007. Temperature corrections for specific gravity hydrometers (15.5ºc/60ºf) Thermophysical properties are measured in low temperature range. Water boiling temperature vs pressure in vacuum table chart. † typical properties, not to be construed as specifications. Freeze protection prevents ice crystal formation at the lowest temperature expected in the coolant circuit. Boiling and freezing point properties of selected substances. Time to freeze piping formulas and calculator. We stated (without offering proof) that this should result in a higher boiling point for the solution compared with pure water.
DSC melting and freezing points of aqueous ethylene glycol with the
How to match glycol levels to various HVAC systems

Ethylene Glycol Freeze Chart

Ethylene Glycol Behaviour at Low Temperature Conditions in

FREEZING POINT DATA FOR AQUEOUS SOLUTIONS OF ETHYLENE GLYCOL (MEG

Glycol Freezing Point Chart

TRZ 4in1 Antifreeze Refractometer Ethylene Glycol, Propylene Glycol
Freezing Point
A Quick Guide to Glycol North Slope Chillers

FREEZING POINT DATA FOR AQUEOUS SOLUTIONS OF ETHYLENE GLYCOL (MEG
Web Ethylene Glycol \(\Left( \Ce{C_2H_6O_2} \Right)\) Is A Molecular Compound That Is Used In Many Commercial Antifreezes.
Specific Heat Should Not Be Indirectly.
40% Mokon Concentration By Volume.
It Is Made Entirely From Food Grade Raw Materials Conforming To Fda Cfr 21 And Nsf Guidelines And Is Acceptable For Use As A Heat Transfer Fluid Where There Is Possibility Of Incidental Food Contact.
Related Post: