Gas Laws Chart
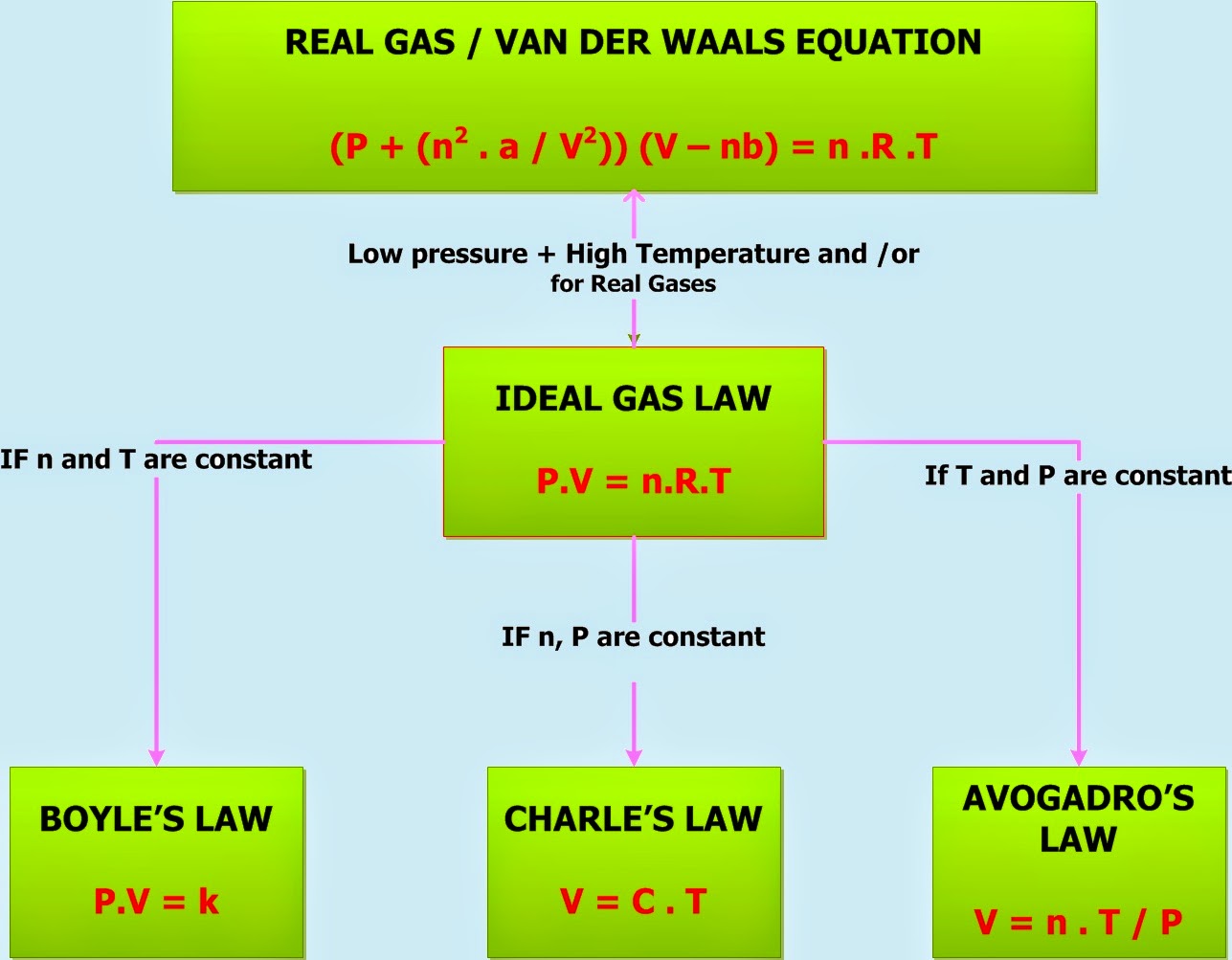
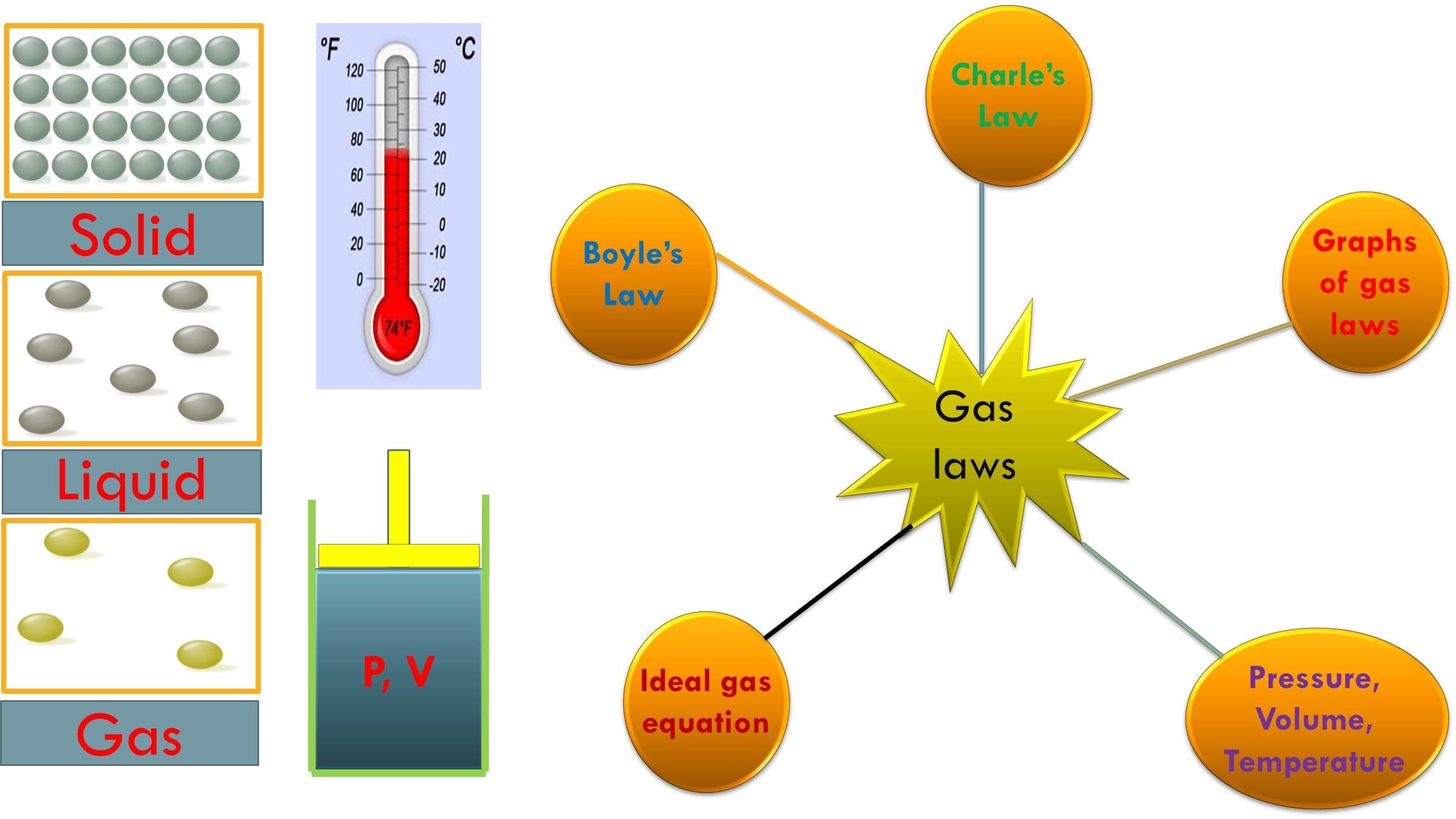
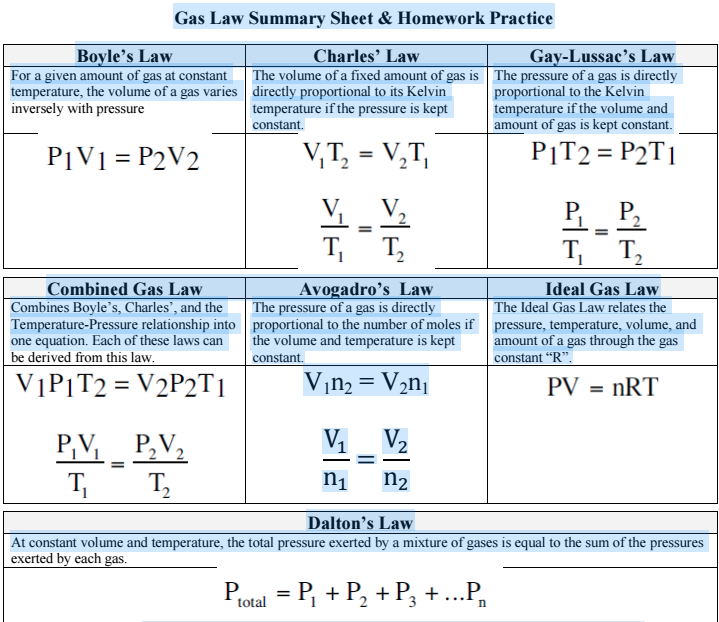
Gas Laws Chart - Web the mathematical modeling of relationships between variables such as the number of gas particles, the temperature of a gas, the volume of the gas container, gas constants, and the pressure of the gas, helps students to progressively expand and derive the gas laws from experimental data. Boyle's law relates a gas's pressure and volume at constant temperature and amount. At constant temperature and pressure, the volume of a sample of gas is directly proportional to the number of moles of gas in the sample. Web the ideal gas law is the equation of state of a hypothetical ideal gas. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. Boyle's law, charles' law, the combined gas law, avogadro's law, and the ideal. This particular gas law ia called boyle's law , after the english scientist robert boyle, who first announced it in 1662. Principle that warm air is less dense than cooler air. The ideal gas law can also relate density and molar mass with the gas constant “r.”. Suppose p is the pressure and v is the volume, then. Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. The gas laws in chemistry are: It states that under a constant temperature when the pressure on a gas increases its volume decreases. The partial pressure of vapor molecules above the surface of a liquid at equilibrium at a given temperature is. In gas laws, temperatures must always be expressed in kelvins. Web p1v1 = p2v2 = constant. At stp, gases have a volume of 22.4 l per. Charles's law relates a gas's volume and temperature at constant pressure and amount. Include units on your work, and write your final answers in the tables. Web the gas laws consist of three primary laws: Web the five gas laws are listed below: While it is important to understand the relationships covered by each law, knowing the originator is not as important and will be rendered redundant once the combined gas law is introduced. And is the ideal gas constant. How do you fill up a. It provides a relationship between the volume occupied by a gas and the absolute temperature. Where , and are the pressure, volume and temperature respectively; Charles' law, boyle's law and avogadro's law (all of which will later combine into the general gas equation and ideal gas law). In 1662, robert boyle systematically studied the relationship between the volume and pressure. Principle that warm air is less dense than cooler air. Robert boyle conducted an experiment on gases to study the deviation of its behaviour in changed physical conditions. Apply boyle’s law using mathematical calculations. The partial pressure of vapor molecules above the surface of a liquid at equilibrium at a given temperature is the vapor pressure (vp) of the liquid. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Web • in chemistry, the relationships between gas physical properties are described as gas laws. In gas laws, temperatures must always be expressed in kelvins. Web the ideal gas law is the equation of state of a. Introduction the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Web as the temperature is raised, the fraction of molecules that can break away into the gas phase grows and vapor pressure increases. Web the ideal gas law relates the four independent physical properties of a gas at any time. Boyle's law, charles'. In 1662, robert boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature. Suppose p is the pressure and v is the volume, then. Include units on your work, and write your final answers in the tables. Apply boyle’s law using mathematical calculations. Some of these properties are pressure, volume,. Typically, these problems aren't so much about doing the math once you. Suppose p is the pressure and v is the volume, then. Principle that warm air is less dense than cooler air. However, a number of rates available to. Introduction the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. While it is important to understand the relationships covered by each law, knowing the originator is not as important and will be rendered redundant once the combined gas law is introduced. Boyle’s law describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. Web coverage of empirical gas laws and the. At constant temperature and pressure, the volume of a sample of gas is directly proportional to the number of moles of gas in the sample. Web a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of a gas: Web the ideal gas law is the equation of state of a hypothetical ideal gas. Web • in chemistry, the relationships between gas physical properties are described as gas laws. Each law is titled by its discoverer. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by august krönig in 1856 [2] and rudolf clausius in 1857. Is the amount of substance; Where , and are the pressure, volume and temperature respectively; Charles' law, boyle's law and avogadro's law (all of which will later combine into the general gas equation and ideal gas law). The ideal gas law can also relate density and molar mass with the gas constant “r.”. Web the gas laws are empirical laws that describe the properties of gases and typically include avogadro's law, boyle's law, and charles' laws and dalton's laws. It provides a relationship between the volume occupied by a gas and the absolute temperature. · try some practice worksheets. Boyle's law, charles' law, the combined gas law, avogadro's law, and the ideal. Web the ideal gas law is often written in an empirical form: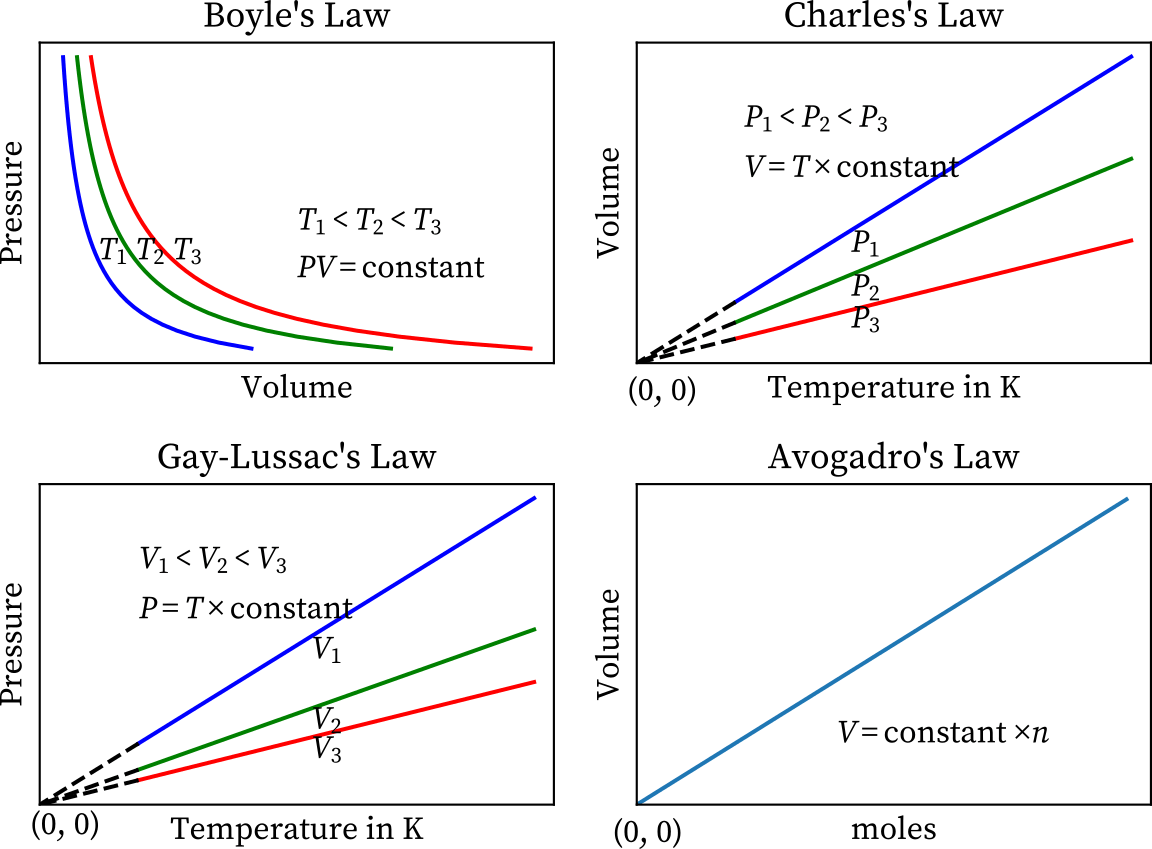
Universal Gas Law Study Guide Inspirit Learning Inc
Gas Laws Ideal Gas Law Chemistry Net

Combined Gas Law — Overview & Calculations Expii

Charle’s vs Boyle’s Gas Laws anchor chart Chemistry classroom
Gas Laws CK12 Foundation
The Gas Laws Definition, Formula & Examples StudiousGuy
Gas Laws Environmental Chemistry

Pin by Joni Loomis on Chemistry (With images) Ideal gas law, Gas laws

The Theories and Behavior of Gas Owlcation

OCR A2 Physics Gas Laws Andrew PoverAndrew Pover
Web European Union Countries Approved A Law On Monday To Impose Methane Emissions Limits On Europe's Oil And Gas Imports From 2030, Pressuring International Suppliers To Cut Leaks Of The Potent.
Web The Ideal Gas Law Relates The Four Independent Physical Properties Of A Gas At Any Time.
At Stp, Gases Have A Volume Of 22.4 L Per.
Include Units On Your Work, And Write Your Final Answers In The Tables.
Related Post:
