How To Draw A Bohr Diagram
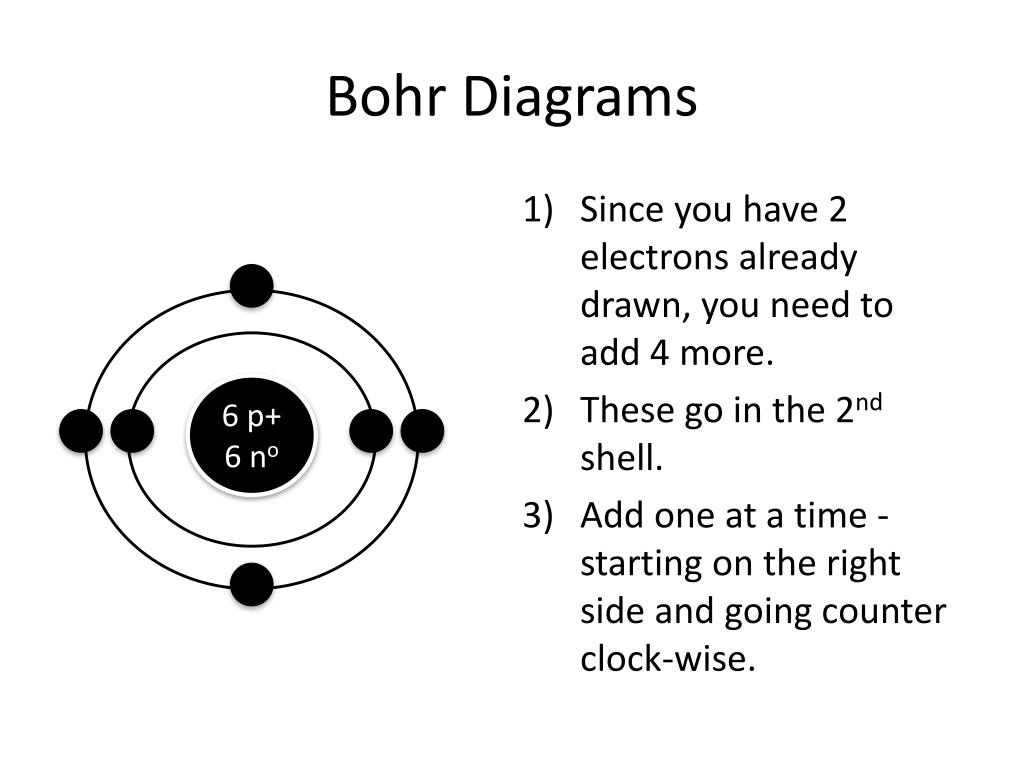
How To Draw A Bohr Diagram - Draw the next shell if you have more electrons to add 6. The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: The protons and neutrons are placed into the nucleus and the. This packet includes a video explaining the bohr model and how to create and use bohr model diagrams, as well as practice problems. Web summary how to draw a bohr model of an atom? 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. The maximum number of electrons that can fill each shell is: Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. The boron family is located in the 13 th group of the periodic table: Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. For example, consider the following diagram for phosphorous: Web bohr diagrams visually depict the distribution of electrons in an atom’s energy levels and orbitals. In order to make a bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. Add your electrons, the 2nd shell can hold up to 8 p:9 n:10 be sure. Web bohr’s model of the atom can be combined with rutherford’s model in diagrams that summarize the numbers and positions of all three subatomic particles. Web part of the series: Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Draw the first shell 4.. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Elements in the 2nd period have two energy levels, and so on. You can effortlessly find every single detail about the elements from this single interactive periodic table. Each energy level is represented by a circle,. The maximum number of electrons that can fill each shell is: The steps to drawing bohr diagrams for elements and. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. Web summary how to draw a bohr model of an atom? • • • find out which period (row) your element. Draw the next shell if you have more electrons to add 6. Web bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Each energy level is represented by a circle, and the orbitals within each level are depicted as individual spaces within the circle. Bohr explained the hydrogen spectrum in terms of. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Discuss how the bohr model can be used to explain atomic spectra. For example, consider the following diagram for phosphorous: Learn how to draw a bohr model with help from an. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. The number of dots equals the number of valence electrons in the atom. Write the number of protons and neutrons in the nucleus 3. Questions tips & thanks want to join the conversation? • • •. In order to make a bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web part. The maximum number of electrons that can fill each shell is: Two in the first shell, eight in the second shell, eight in the third shell. Questions tips & thanks want to join the conversation? The steps to drawing bohr diagrams for elements and. Draw the first shell add the electrons, the first shell can only hold 2 5. The protons and neutrons are placed into the nucleus and the. Web bohr’s model of the atom can be combined with rutherford’s model in diagrams that summarize the numbers and positions of all three subatomic particles. You can effortlessly find every single detail about the elements from this single interactive periodic table. Elements in the 2nd period have two energy. Web in the bohr model, there are a few rules that will help you draw accurate diagrams. This is how many electrons you will draw. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. Questions tips & thanks want to join the conversation? The number of dots equals the number of valence electrons in the atom. For example, consider the following diagram for phosphorous: Draw the next shell if you have more electrons to add 6. E ( n) = − 1 n 2 ⋅ 13.6 ev. You will get the detailed information about the periodic table which will convert a newbie into pro. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Write the number of protons and neutrons in the nucleus 3. Describe the arrangement of electrons using the shell model. To evaluate the number of valence electrons using a bohr model. Discuss how the bohr model can be used to explain atomic spectra. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels.
How to Draw Bohr Rutherford Diagrams YouTube
Bohr Model of the Atom Overview and Examples
How to Draw Bohr Diagrams Presentation Chemistry
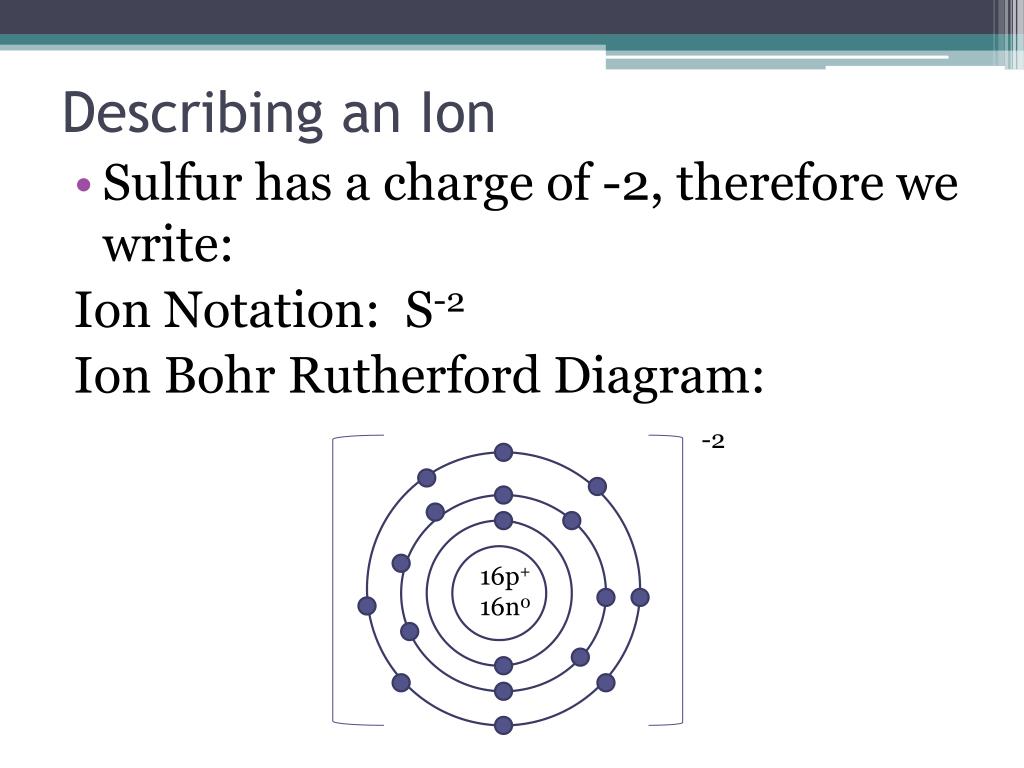
How To Draw A Bohr Model For An Ion

Bohr's Atomic Model — Overview & Importance Expii
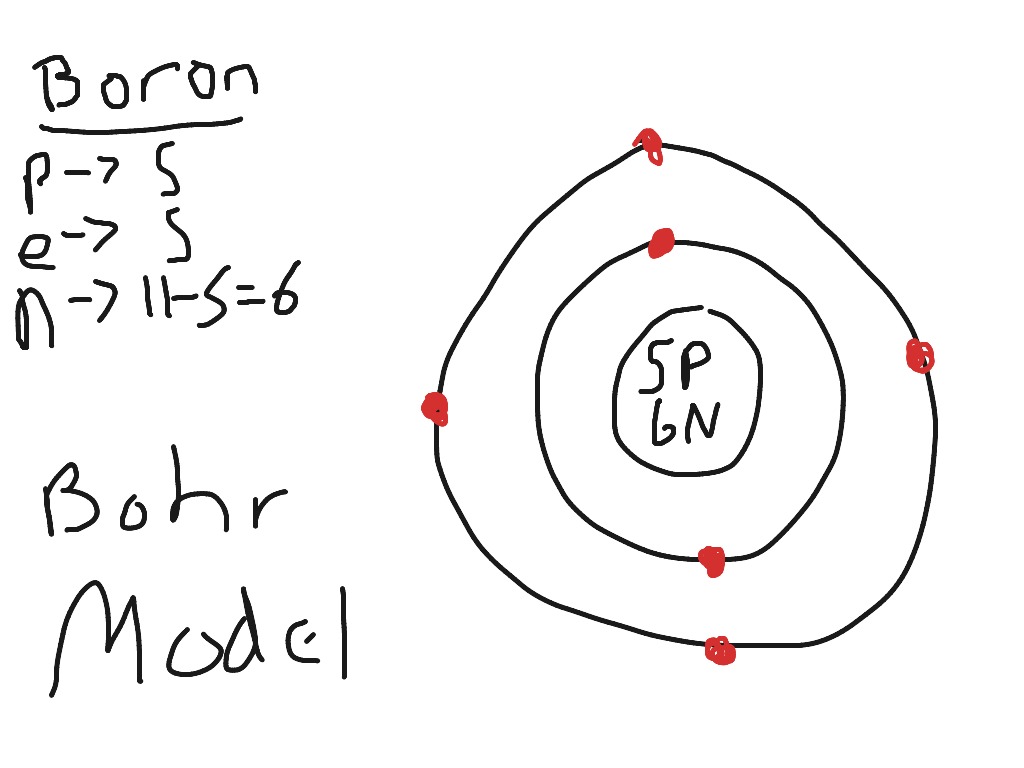
Boron Bohr model Science ShowMe
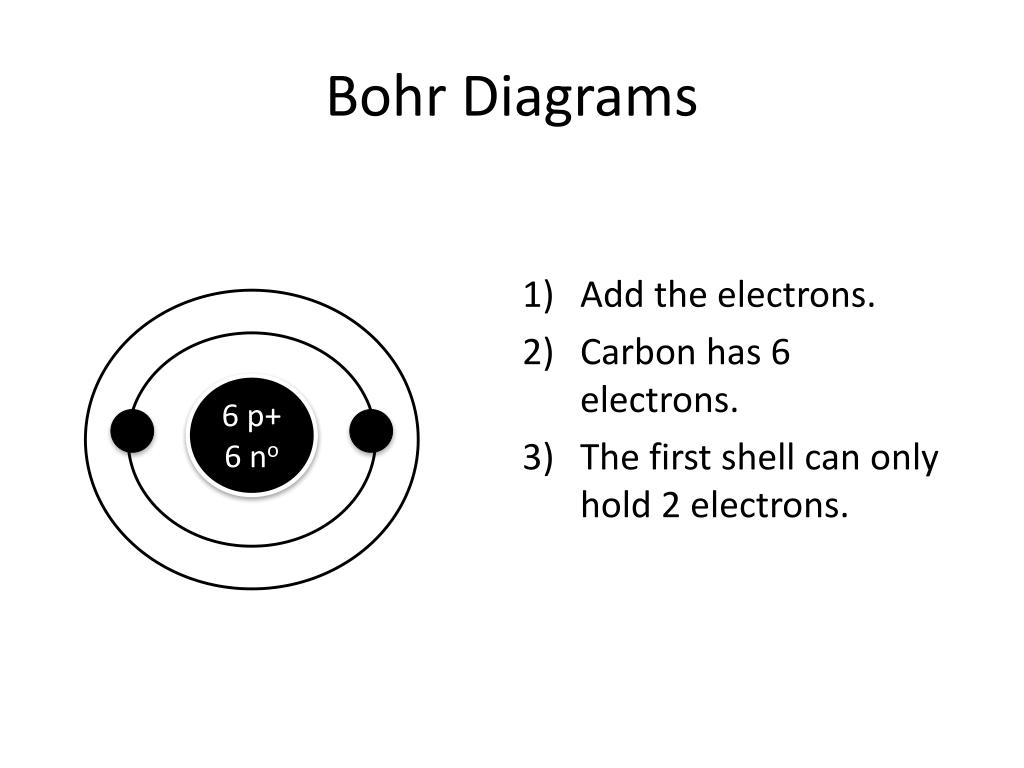
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
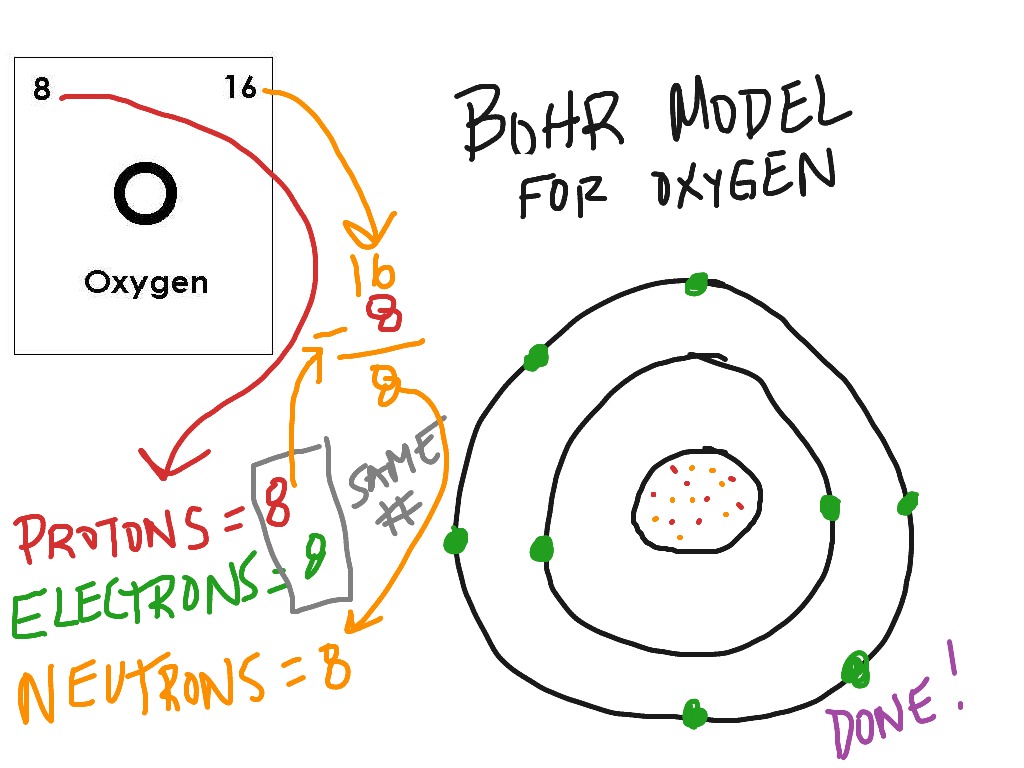
Bohr Model Oxygen Science ShowMe
How to Draw Bohr Diagrams Presentation Chemistry
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
Web Bohr’s Model Of The Atom Can Be Combined With Rutherford’s Model In Diagrams That Summarize The Numbers And Positions Of All Three Subatomic Particles.
• • • Find Out Which Period (Row) Your Element Is In.
You Will Also Learn How To Write Bohr Model Electron Configuration As.
This Packet Includes A Video Explaining The Bohr Model And How To Create And Use Bohr Model Diagrams, As Well As Practice Problems.
Related Post:
.PNG)
.PNG)