How To Draw A Bohr Model
How To Draw A Bohr Model - 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on. Discuss how the bohr model can be used to explain atomic spectra. Web britannica quiz matter and more quiz how does niels bohr's atomic model work? E ( n) = − 1 n 2 ⋅ 13.6 ev. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Find the number of protons, electrons, and neutrons of an atom. Two in the first shell, eight in the second shell, eight in the third shell. Web define an energy level in terms of the bohr model. You will get the detailed information about the periodic table which will convert a newbie into pro. Learn how to draw a bohr model with help from an artist in this free. This packet includes a video explaining the bohr model and how to create and use bohr model diagrams, as well as. Bohr explained the hydrogen spectrum in terms. Web bohr’s model revolutionized the understanding of the atom but could not explain the spectra of atoms heavier than hydrogen. Write the number of protons and neutrons in the nucleus 3. The electron moves around the nucleus in circular orbits that can have only certain allowed radii. Nobel prize in physics, 1922), proposed a theoretical model for the hydrogen atom. Draw the nucleus of an atom. Write the number of protons and neutrons at the center of the nucleus. Write the number of protons and neutrons in the nucleus 3. Find the number of protons, electrons, and neutrons of an atom. You will get the detailed information about the periodic table which will convert a newbie into pro. Bohr’s model required only one assumption: You will get the detailed information about the periodic table which will convert a newbie into pro. Key concepts electrons can occupy only certain regions of space, called orbits. To evaluate the number of valence electrons using a bohr model. Web this chemistry tutorial video walks you through how to draw a bohr model,. Models help us visualize atomic structure. See all videos for this article bohr model of the atom in the bohr model of the atom, electrons travel in defined circular orbits around the nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. Web in the bohr model,. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Electrons must occupy the lowest available shell, closest to the nucleus. Find out which period (row) your element is in. See all videos for this article bohr model of the atom in the bohr model of the atom,. Electrons must occupy the lowest available shell, closest to the nucleus. The electron moves around the nucleus in circular orbits that can have only certain allowed radii. Draw the nucleus of an atom. Web britannica quiz matter and more quiz how does niels bohr's atomic model work? Write the number of protons and neutrons in the nucleus 3. Draw the nucleus of an atom. Discuss how the bohr model can be used to explain atomic spectra. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Models help us visualize atomic structure. The steps to drawing bohr diagrams for. Web how to draw bohr diagrams. So, we represent atoms using models. Write the number of protons and neutrons in the nucleus 3. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit. Find the number of protons, electrons, and neutrons of an atom. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. See all videos for this article bohr model of the atom in the bohr model of the atom, electrons travel in defined circular orbits around the nucleus.. This is how many electrons you will draw. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. The protons and neutrons are placed into the nucleus and the. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Web how to draw bohr diagrams. E ( n) = − 1 n 2 ⋅ 13.6 ev. Two in the first shell, eight in the second shell, eight in the third shell. Add your electrons, the 2nd shell can hold up to 8 p:9 n:10 be sure to add them one at a time going clock wise. Draw the next shell if you have more electrons to add 6. Write the number of protons and neutrons in the nucleus 3. See all videos for this article bohr model of the atom in the bohr model of the atom, electrons travel in defined circular orbits around the nucleus. Nobel prize in physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Write the number of protons and neutrons at the center of the nucleus. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright.
How to draw a Bohr model of an atom BohrRutherford Diagrams
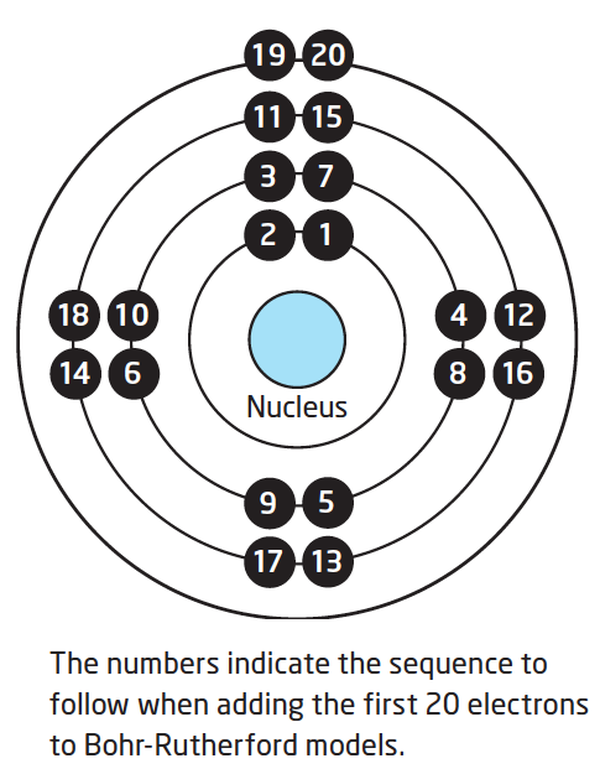
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
Bohr Model of the Atom Overview and Examples
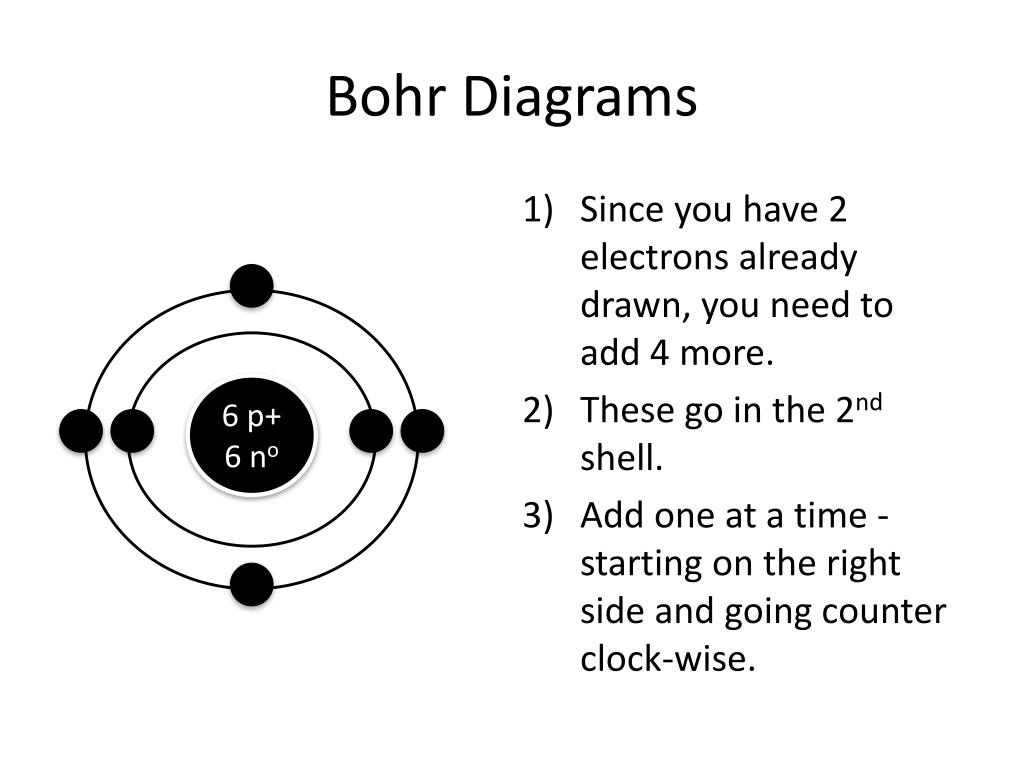
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
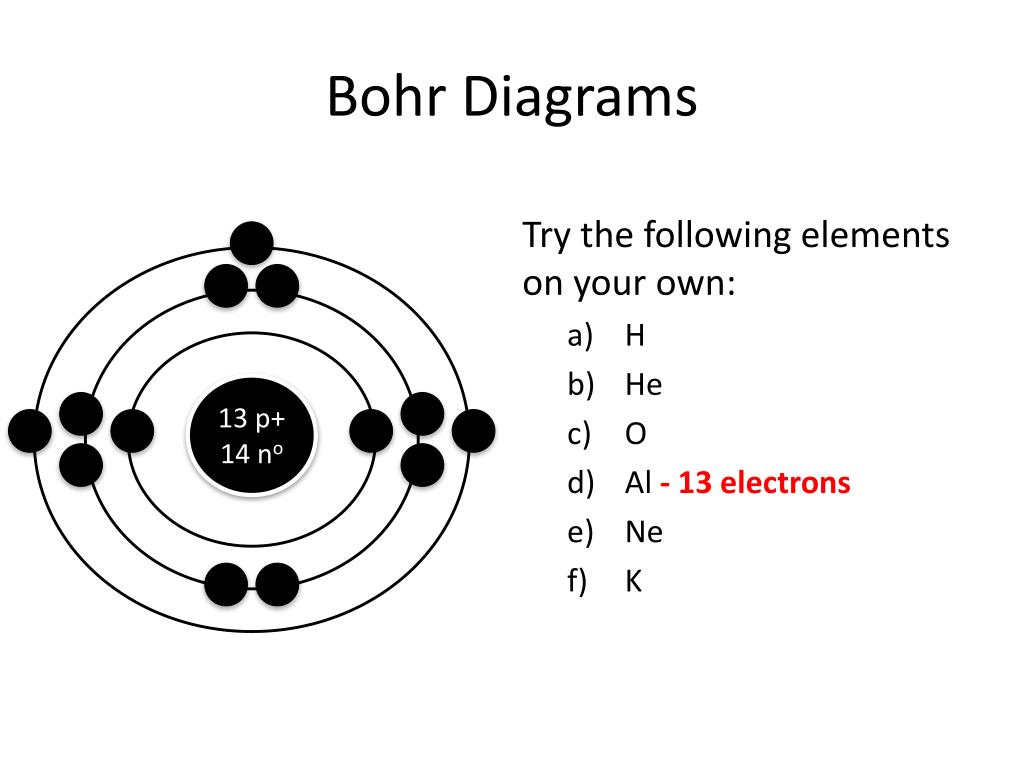
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
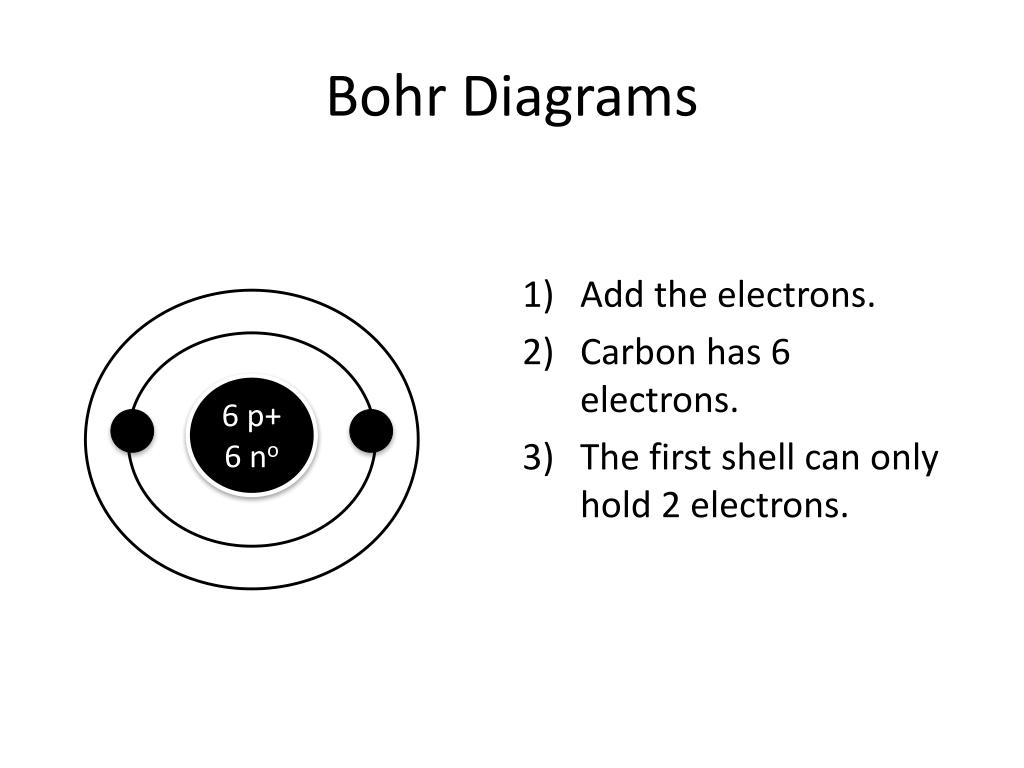
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
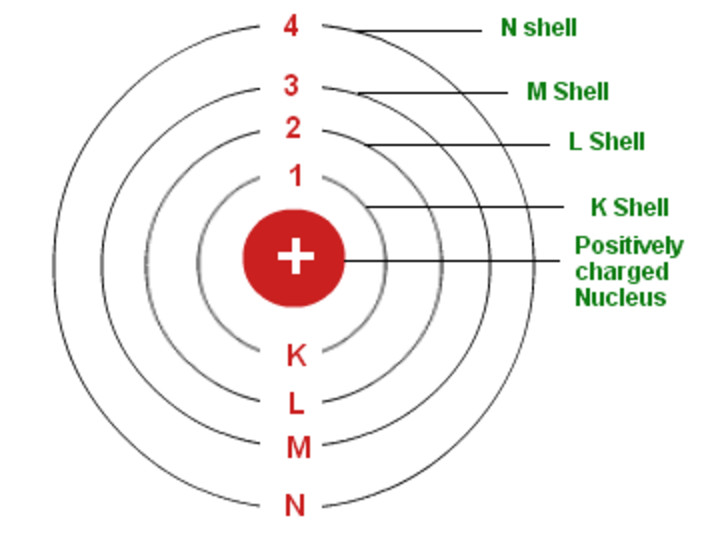
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom

How to Draw a Bohr Model Step by Step Easy YouTube
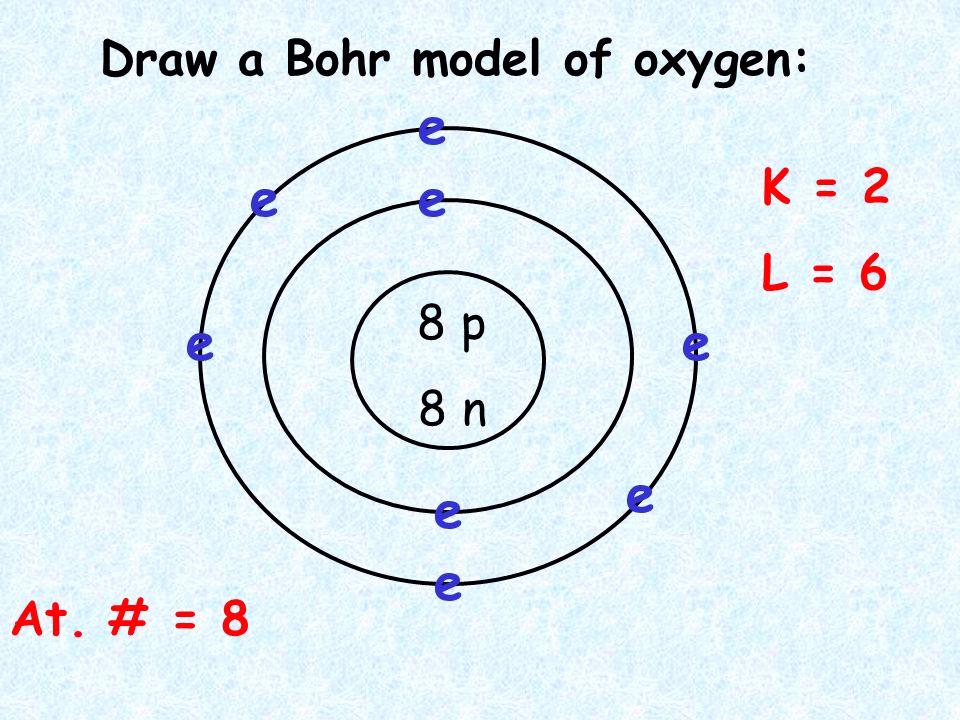
Diagram Bohr Model Periodic Table Periodic Table Timeline
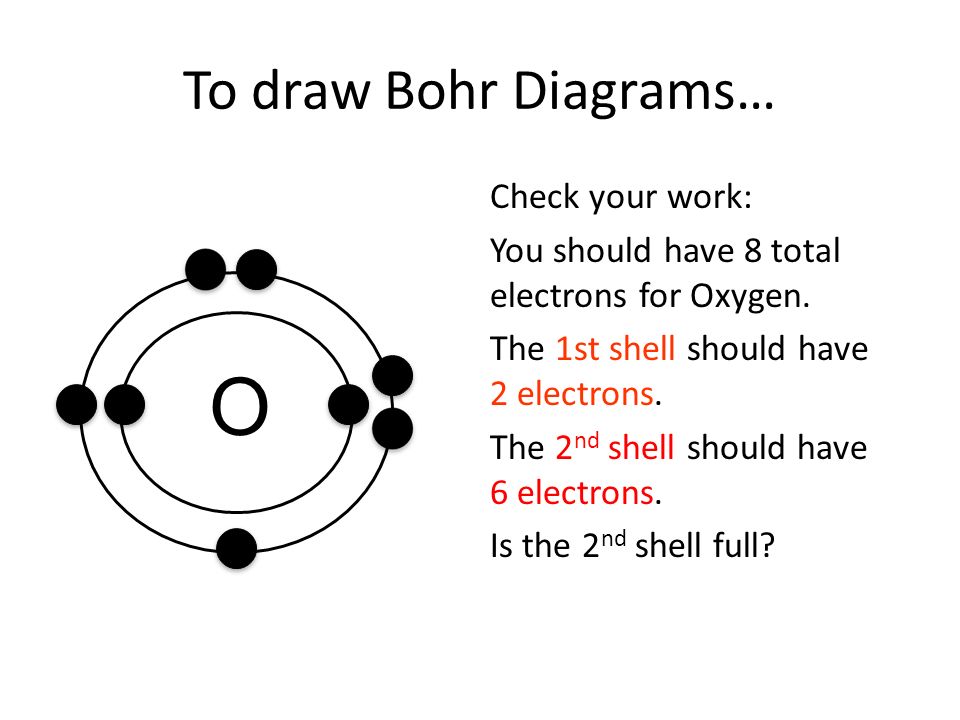
How To Draw A Bohr Model For An Ion Riset
Find Out Which Period (Row) Your Element Is In.
The Maximum Number Of Electrons That Can Fill Each Shell Is:
Web In The Bohr Model, There Are A Few Rules That Will Help You Draw Accurate Diagrams.
This Packet Includes A Video Explaining The Bohr Model And How To Create And Use Bohr Model Diagrams, As Well As.
Related Post: