How To Draw Amino Acids At Different Ph
How To Draw Amino Acids At Different Ph - In a reverse reaction, the peptide bond can be cleaved by water (hydrolysis). 22k views 8 years ago a2 amino. By polytechnic university of milan. Twenty different amino acids are used to build a protein. Web let's learn how to deduce the structure of amino acid at different ph using aspartic acid as an example. The exact opposite would happen for protonation of amino acids. What is the range of ph values when it will be positively charged? Web new study explores amino acid that turns into gel in water. Web the 20 amino acids below are foundational to human metabolic processes and can be combined in a variety of ways to build different proteins. Protein may contain one or. An amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. If we manipulate the ph p h, for example, by adding a strong. #pk_a# values for amino acids. Web draw the predominant form of a given amino acid in a solution of known ph,. An amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. The basic structure of an amino acid is shown to the right. A 1.0 −m 1.0 − m solution of a simple carboxylic acid like acetic acid has a ph p h of ∼. Amino (−nh3+) and carboxylate (−coo−) and an organic side chain. Use the following link to find a list of the #pk_a# values for all the amino acids. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. Amino acids are amphoteric which means they can act as an acid or a base. Web a typical amino. Pka1 for alpha acid = 1.88. How to draw the structure of a basic amino acid at any ph. There are twenty common amino acids, which differ in the r group attached to the central carbon atom. Web new study explores amino acid that turns into gel in water. In this reaction, water is released. What is the range of ph values when it will be negatively charged? If the solution is more alkaline than acid fg (ph > pka), the acid. The r group varies among amino acids and determines the differences between these protein monomers. Starting with the amine end, write nh3+ and from there, draw a line up to the right, creating. Web there are 20 common amino acids found in proteins, each with a different r group (variant group) that determines its chemical nature. Pka1 for alpha acid = 1.88 pka2 for r group acid = 3.65 pka3 for alpha amine = 9.60 at a. A 1.0 −m 1.0 − m solution of a simple carboxylic acid like acetic acid has. This video shows how ph can effect protein structure and how to determine the structure from knowing the pka of functional groups. The exact opposite would happen for protonation of amino acids. Each amino acid has its own pi value based on the properties of the amino acid. Web effect of ph and pka on amino acid structure. Web microalgae. 1.3k views 2 years ago introductory organic and. Structure of acidic amino acids at various ph. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Web draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. This video shows how. Web for the amino acids with protonated r groups, you need to pay attention to their #pk_a# values. How to draw the structure of a basic amino acid at any ph. At a certain ph, we compare the ph of solution against pka of each functional group. Web effect of ph and pka on amino acid structure. Web a typical. Web the structure of an amino acid allows it to act as both an acid and a base. If the ph is higher (in alkaline conditions) than the isoelectric point then the amino acid acts as an acid and donates a proton from its carboxyl group. The basic structure of an amino acid is shown to the right. Then from. We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Each amino acid has its own pi value based on the properties of the amino acid. Web proteins, being composed of amino acids, exhibit characteristics related to their charge at different ph levels. 22k views 8 years ago a2 amino. There are twenty common amino acids, which differ in the r group attached to the central carbon atom. Web google classroom about transcript the isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. Web a typical amino acid with one amino group and one carboxylic acid group, such as serine can exist in water in several iconic forms. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Web microalgae are powerful source for nutritionally valuable components as proteins, carbohydrates and especially unsaturated fatty acids. Pka1 for alpha acid = 1.88 pka2 for r group acid = 3.65 pka3 for alpha amine = 9.60 at a. At ph values above or below the isoelectric point, the molecule will have a net charge which depends on its pi value as well as the ph of the solution in which the amino acid is found. Pka2 for r group acid = 3.65. What is the range of ph values when it will be negatively charged? Web let's learn how to deduce the structure of amino acid at different ph using aspartic acid as an example. Identify whether it is polar, nonpolar, acidic, or basic. Then from c1, draw a line down and to the right to create another point called c2.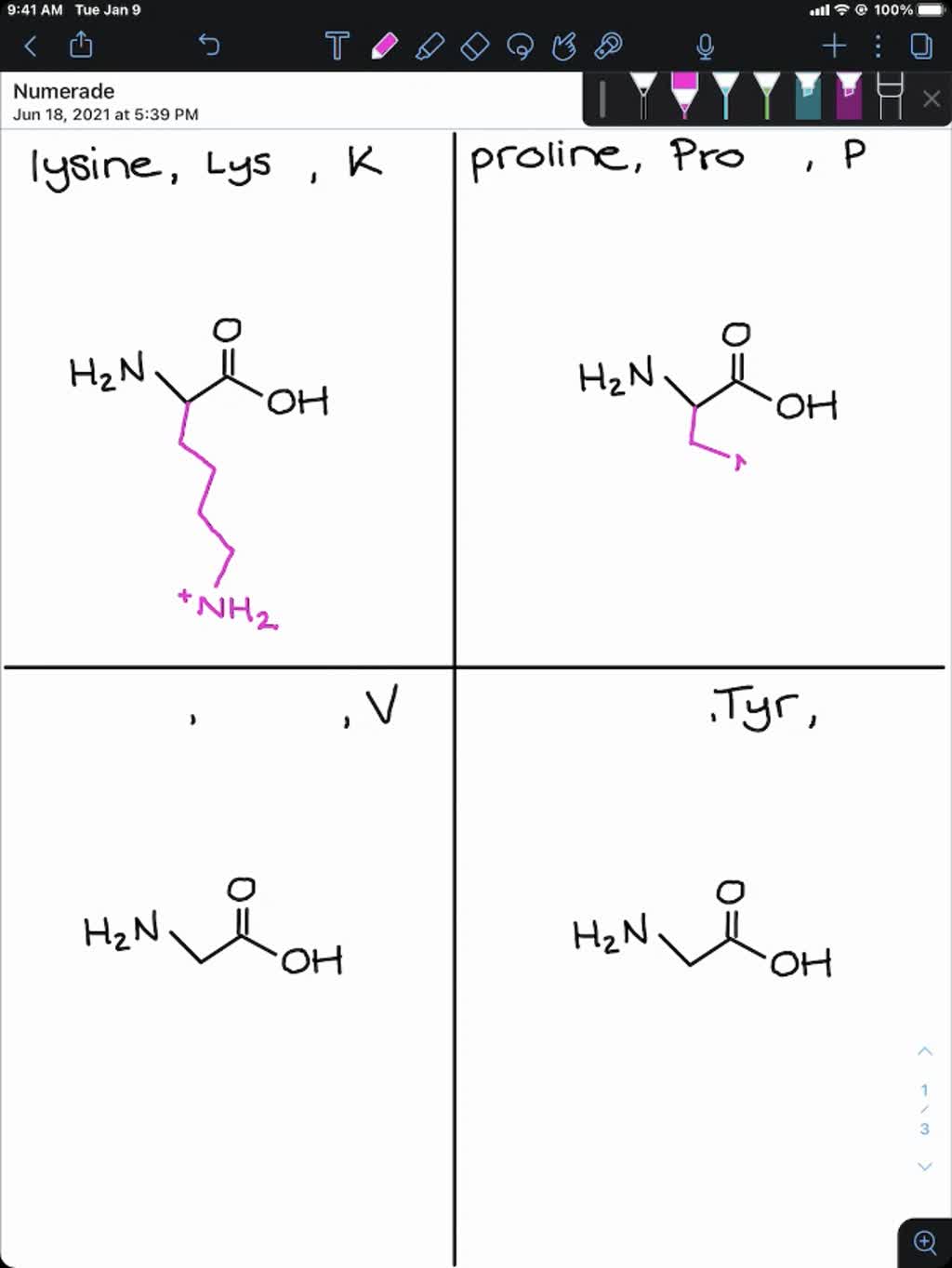
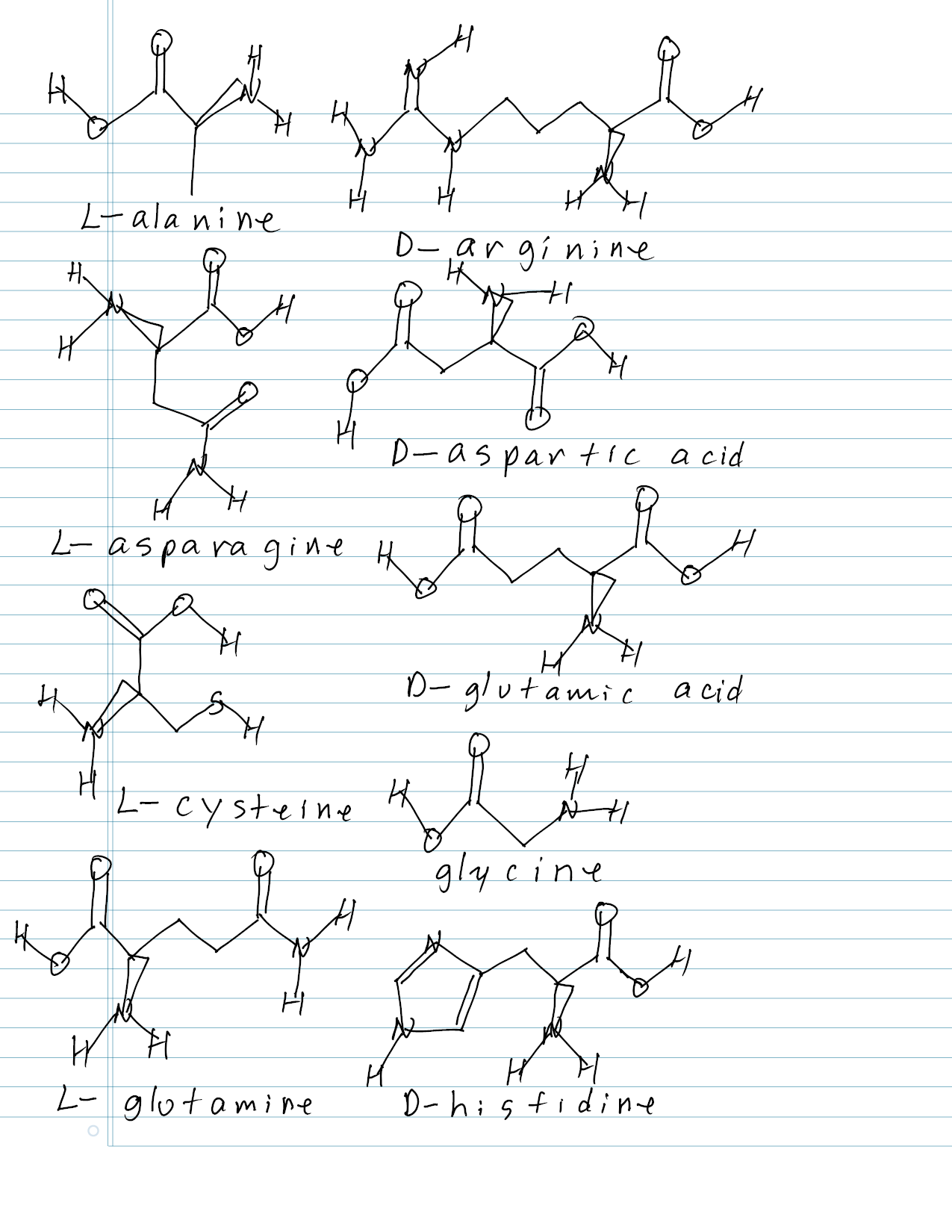
SOLVEDDraw the structure for each of the following amino acids at
Amino Acid Classification and Structure ( Read ) Chemistry CK12

MCAT Biology & Biochemistry Amino Acid Structures Part II ditki
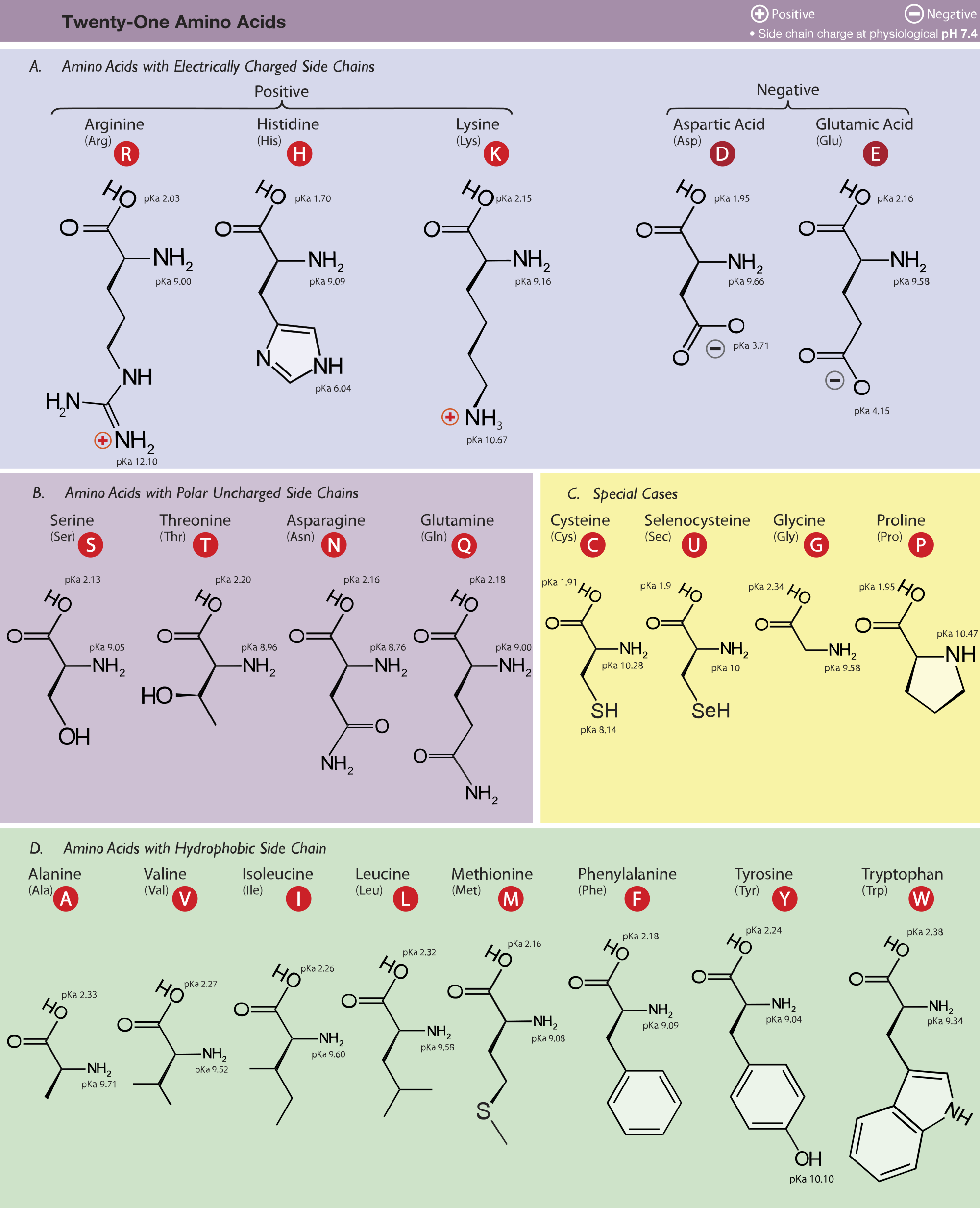
Amino Acid Study Guide Structure and Function Albert.io

How To Draw Amino Acids Structures Learn Mnemonics YouTube

69 Structure of basic amino acids at various pH YouTube
Amino Acids

68 Structure of acidic amino acids at various pH YouTube
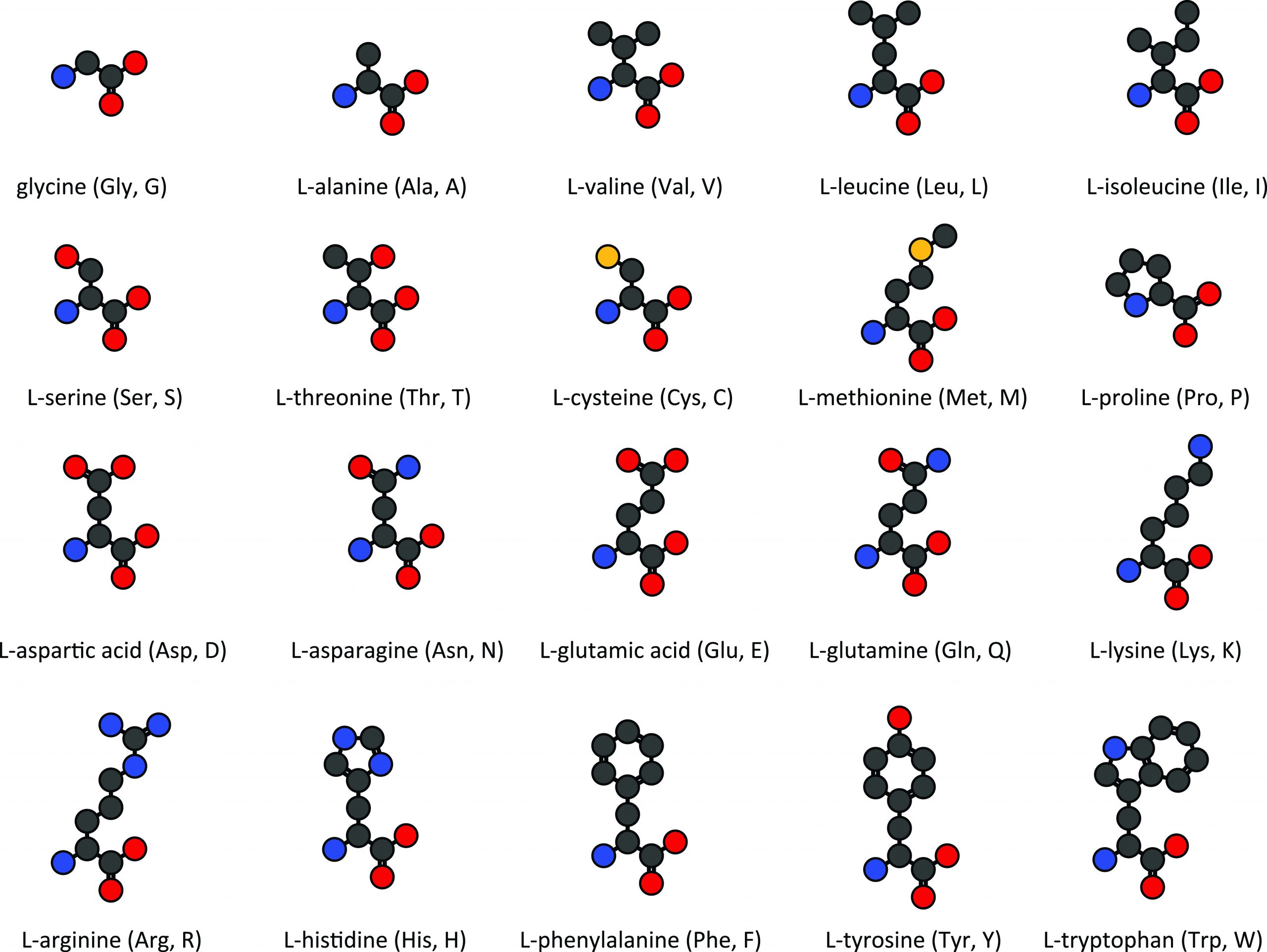
Amino acids. 2D chemical structures of the 20 common amino acids REP ONE
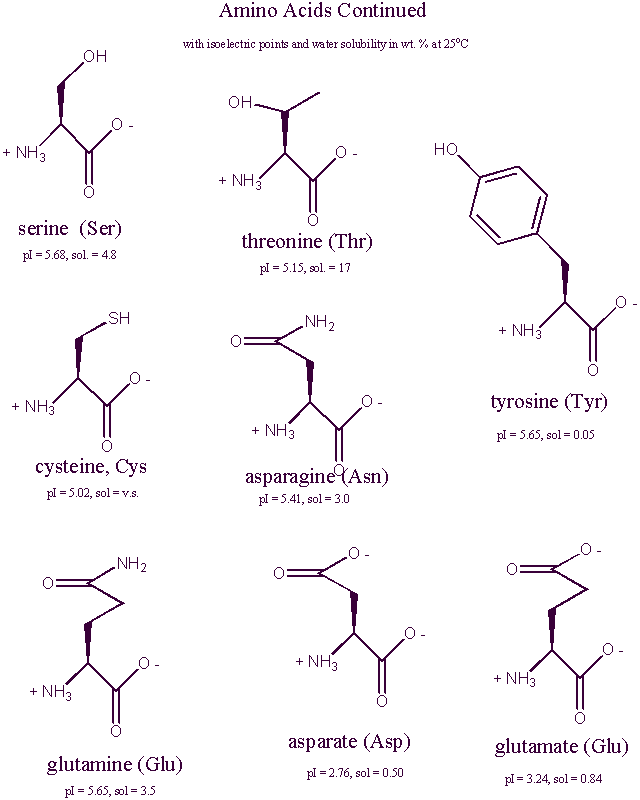
Amino Acid Structures at Physiological pH
If We Manipulate The Ph P H, For Example, By Adding A Strong.
Twenty Different Amino Acids Are Used To Build A Protein.
1.3K Views 2 Years Ago Introductory Organic And.
Web This Gives It A Positive Change.
Related Post:
