How To Draw Atom Structure
How To Draw Atom Structure - (generally, the least electronegative element should be placed in the center.) connect each atom to the central atom with a single bond (one electron pair). Then play a game to test your ideas! Web the commonest way to draw structural formulae. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web eight in the third shell. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Each shell is separated by a full stop or a comma. The number of dots equals the number of valence electrons in the atom. Neutrons are simply equal to the atomic mass minus the number of protons. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Each element has its own atomic number, which is equal to the number of protons in its nucleus. Web 0:00 / 3:16 how to draw an atom |. Atoms themselves are composed of protons, neutrons, and electrons. In the shorthand notation for electron configuration, the number of electrons in each shell can be written rather than drawn. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Shared pairs of electrons are drawn as lines between atoms, while lone. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Atoms themselves are composed of protons, neutrons, and electrons. Web 0:00 / 3:16 how to draw an atom | atom structure diagram | physics project diagram kids drawing practice 100k subscribers subscribe 1.6k views 10 months ago how. To indicate they are protons, draw them as circles with plus signs contained inside. Each shell is separated by a full stop or a comma. Web a lewis structure is a graphic representation of the electron distribution around atoms. Web hello friends,i am namrata, a drawing teacher. The final ring or shell of electrons contains the typical number of valence. Neutrons are simply equal to the atomic mass minus the number of protons. Web hello friends,i am namrata, a drawing teacher. Each element has its own atomic number, which is equal to the number of protons in its nucleus. Web 0:00 / 3:16 how to draw an atom | atom structure diagram | physics project diagram kids drawing practice 100k. Erase the c in the center circle, and draw in your protons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic table tiles. The numbers of subatomic particles. Therefore,. The electron shells are shown, moving outward from the nucleus. Web 0:00 / 3:16 how to draw an atom | atom structure diagram | physics project diagram kids drawing practice 100k subscribers subscribe 1.6k views 10 months ago how to draw an atom |. Add enough electrons (dots) to the outer atoms to. To indicate they are protons, draw them. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. Many of our lessons are. Calcium, the 20 th element, has two further electrons that go in the fourth shell. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.. The atomic number of an element describes the total number of protons in its nucleus. The number of dots equals the number of valence electrons in the atom. Each shell is separated by a full stop or a comma. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Add enough electrons (dots) to the outer atoms to. Web hello,,,today, we are learning how to draw atom structure easy tutorial learn how to draw the easy, step by step, while having fun and building skills and confidence. Web here are the. Erase the c in the center circle, and draw in your protons. A lewis structure also helps to make a prediction about the geometry of a molecule. Calcium, the 20 th element, has two further electrons that go in the fourth shell. Web hello friends,i am namrata, a drawing teacher. The atomic number of an element describes the total number of protons in its nucleus. The electron shells are shown, moving outward from the nucleus. Web primarily, the atomic structure of matter is made up of protons, electrons and neutrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. Web draw your protons and neutrons. Web eight in the third shell. Add enough electrons (dots) to the outer atoms to. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. (generally, the least electronegative element should be placed in the center.) connect each atom to the central atom with a single bond (one electron pair).
Learn the Parts of an Atom

Draw A Simple Diagram Of An Atom Labeled Protons Neutrons And Electrons
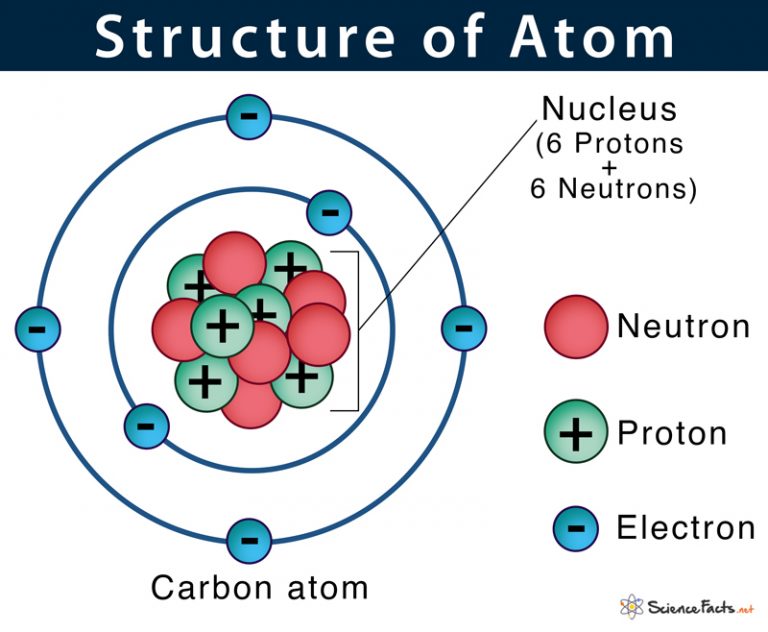
Atom Definition, Structure & Parts with Labeled Diagram

How to Draw an Atom Really Easy Drawing Tutorial
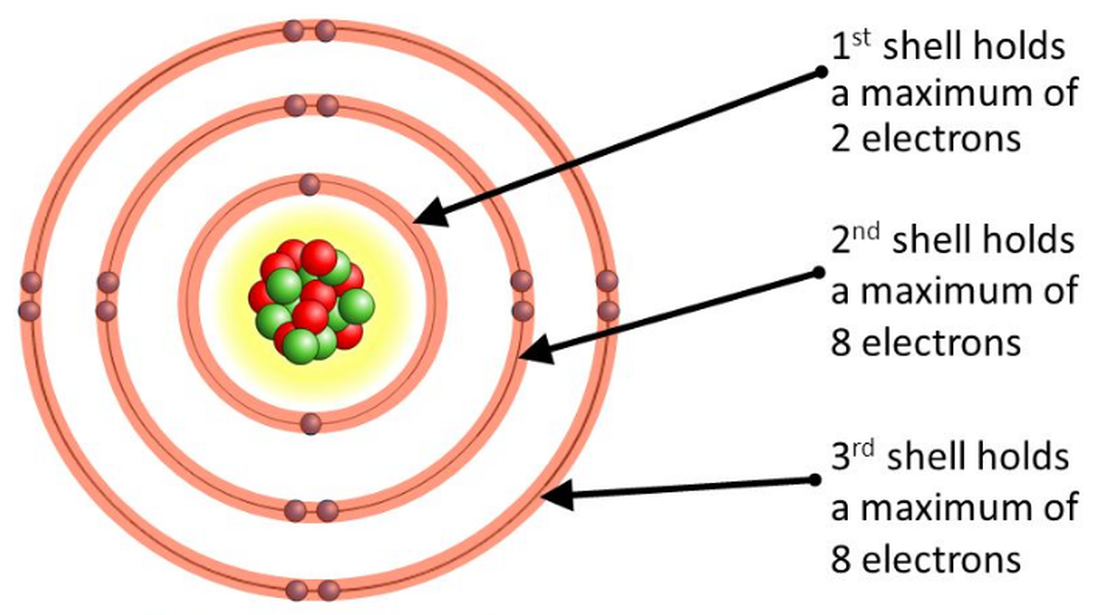
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

How to Draw an Atom Really Easy Drawing Tutorial
How to Draw an Atom Step by Step Simple and Easy YouTube

Atom Definition, Structure & Parts with Labeled Diagram

Drawing Atoms Montessori Muddle
How to draw an ATOMIC structure YouTube
Each Shell Is Separated By A Full Stop Or A Comma.
You Can Simplify The Formula By Writing, For Example, Ch 3 Or Ch 2 Instead Of Showing All These Bonds.
The Final Ring Or Shell Of Electrons Contains The Typical Number Of Valence Electrons For An Atom Of That Element.
Each Element Has Its Own Atomic Number, Which Is Equal To The Number Of Protons In Its Nucleus.
Related Post: