How To Draw D Orbitals
How To Draw D Orbitals - For an s orbital, draw a circle; To draw an atomic orbital, start by drawing a circle to represent the nucleus of the atom. P orbital contains 3 boxes that can hold a maximum of 6 electrons. 1s 2 2s 2 2p 1. Web what are orbitals? Web easy way to draw d orbitals uma's saastra vijnan science is life 108 subscribers subscribe 28 share save 1.7k views 2 years ago in this vedio i gave tips to remember d orbital's shape.d. Then, draw the appropriate wave function for the orbital. S orbital contains 1 box that can hold a maximum of 2 electrons. Learn all of this in this lesson with examples at the end. This is also due to the history when they were discovered. Web d orbitals (l=2) subshells with l = 2 have five d orbitals; Web four orbitals have six lobes oriented in various planes (easiest to draw). The five equivalent 3d orbitals of the hydrogen atom. Web how to draw orbital diagrams the science classroom 58.5k subscribers join subscribe subscribed 1.7k 363k views 10 years ago chemistry tutorials: Below are representations. S orbital contains 1 box that can hold a maximum of 2 electrons. The average energy of the five d orbitals is the same as for a spherical distribution of a −6. Web four orbitals have six lobes oriented in various planes (easiest to draw). The five d orbitals have m l values of −2, −1, 0, +1, and +2.. Web what are orbitals? At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z). The five d orbitals have m l values of −2, −1, 0, +1, and +2. The first principal shell to have a d subshell corresponds. Generally, there are three types of bonding and antibonding interactions that may occur with d d orbitals: Electron configurations electron configurations are expressed through a notation that looks like this: Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. In addition to s and p orbitals, there are two other sets of orbitals which. Web d orbitals (l=2) subshells with l = 2 have five d orbitals; Web an orbital is a space where a specific pair of electrons can be found. The five d orbitals have m l values of −2, −1, 0, +1, and +2. Then, draw the appropriate wave function for the orbital. An s orbital is a sphere. An s orbital is a sphere. They present a challenge to draw in three dimensions, so it is much easier to draw the “between” ones only in two. You will note that the 3 d orbits have two nodal surfaces. Web in transition metals and other heavier elements, the d d orbitals may combine with other orbitals of compatible symmetry. Learn all of this in this lesson with examples at the end. The average energy of the five d orbitals is the same as for a spherical distribution of a −6. To draw an atomic orbital, start by drawing a circle to represent the nucleus of the atom. Once again we are looking at ones that are defined by the. You will note that the 3 d orbits have two nodal surfaces. This lesson particularly revolves around o and a level. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. The five equivalent 3d orbitals of the hydrogen atom. You should, however, be aware. 1s 2 2s 2 2p 1. Below are representations of the d. This lesson particularly revolves around o and a level. You should, however, be aware that there are five possible orientations for d orbitals. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the aufau principle to order the orbitals and hence. Sigma ( σ σ ), pi ( π π ), and delta ( δ δ) bonds. The first principal shell to have a d subshell corresponds to n = 3. D x y, d y z, d z x, d x 2 − y 2 a n d d z 2 was this answer helpful? The five equivalent 3d orbitals. Web four orbitals have six lobes oriented in various planes (easiest to draw). S orbital contains 1 box that can hold a maximum of 2 electrons. Web the 5s, 5p, and 5d orbitals are the most complex of all. Web you can puzzle them out from the rotating images, the dot density diagrams and the orbital surface diagrams if you like, but analysis of these orbitals is usually considered beyond the scope of general chemistry. Web key concepts an orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. This lesson particularly revolves around o and a level. In two dimensions, we draw it as a circle. Of the four, s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the aufau principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s → 2s → 2p x 2p y 2p z → 3s → 3p x Web orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to represent the spins of the electrons too. The five equivalent 3d orbitals of the hydrogen atom. Sigma ( σ σ ), pi ( π π ), and delta ( δ δ) bonds. Web an orbital is a space where a specific pair of electrons can be found. Generally, there are three types of bonding and antibonding interactions that may occur with d d orbitals: Below are representations of the d. Mdcat chemistry | mdcat chemistry tricks | how to prepare for.
draw the shape of d orbitals Brainly.in

1.The five different d atomic orbitals. The figure is adapted from

How To Draw Orbitals Deepcontrol3

Top Notch Tips About How To Draw Orbital Diagrams Spellquestion

how to draw shapes of d orbitals elliottlyde
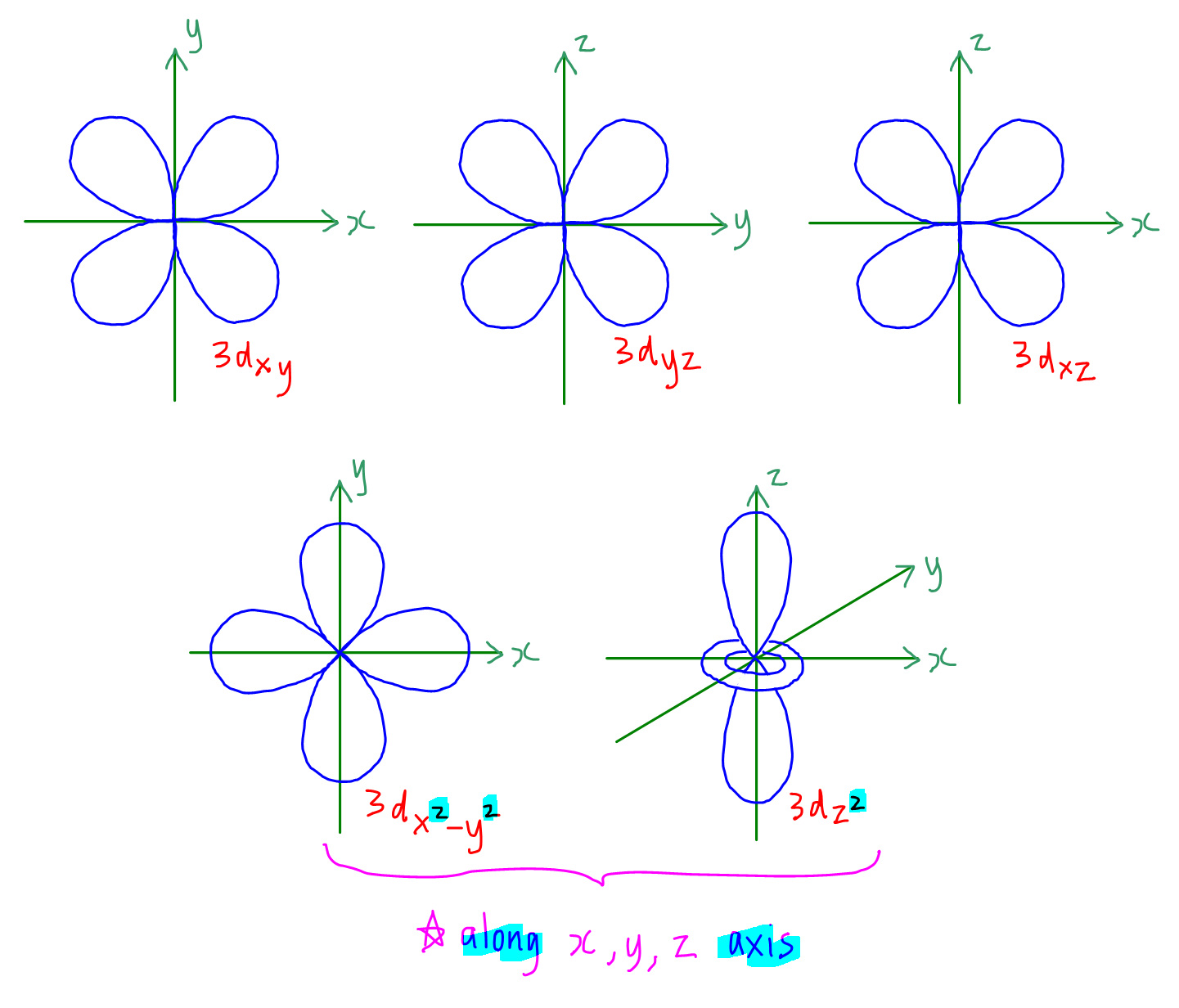
How to Draw Shapes of Orbitals

How To Draw Shapes Of D Orbitals at How To Draw
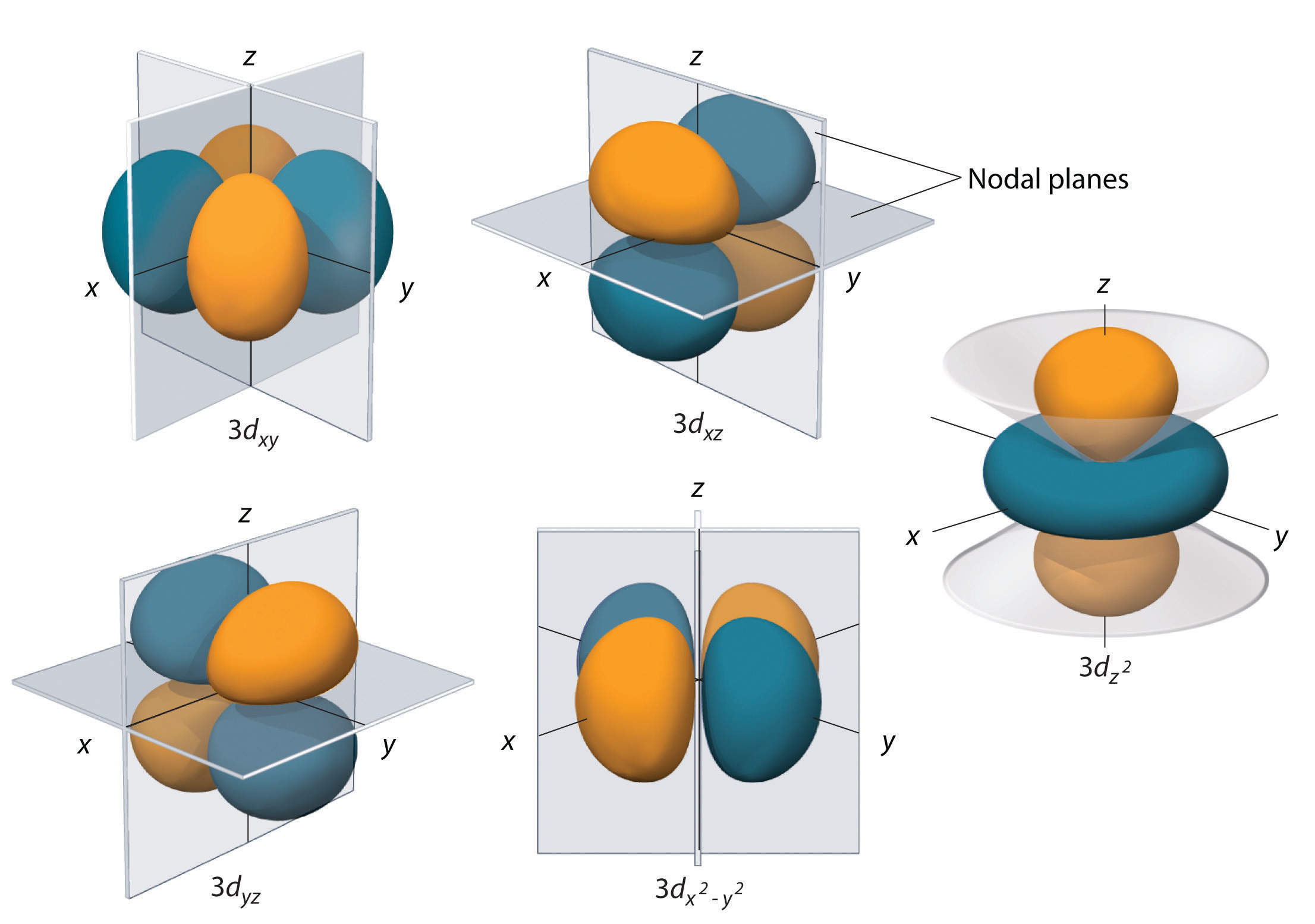
6.6 3D Representation of Orbitals Chemistry LibreTexts

Shapes of Atomic Orbitals Shape of s, p, d, f Orbitals, FAQs, Examples

how to draw shapes of d orbitals
Orbitals Can Be Represented As Boxes With The Electrons Depicted With Arrows.
13 Similar Questions Q 1 Draw Orbit Structure Diagram Of (A) Sodium Chloride (Nacl).
We Classified The Different Orbital Into Shells And Sub Shells To Distinguish Them More Easily.
Once Again We Are Looking At Ones That Are Defined By The Cartesian Coordinate System.
Related Post: