How To Draw Orbital Diagram
How To Draw Orbital Diagram - Web in this article, we leave discuss all the different rules and corporate of filling electrons in an atomic oscillated diagram, followed by plenty of examples to drawing certain orbital diagram oder orbital notation configuration. Web typically, they only show the outermost electrons. Imagine shells around the nucleus, that get bigger and bigger. Simple cubic unit cell 2m. The first one being the auf bau principle, the auf bau principle states that each electron occupies the lowest energy orbital available. Web orbital diagrams are a pictorial description of electrons in an atom. Start by drawing a square to represent the 1s orbital. Orbital diagrams must follow 3 rules: Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Web learn how to draw orbital diagrams. Orbital diagrams must follow 3 rules: This article will explore the basics of how to draw each type of diagram, and important rules to follow in their construction. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Web a p orbital along the. Web typically, they only show the outermost electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. Atomic, ionic, and molecular solids 5m. Molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. The first one being the auf bau principle, the. This is also due to the history when they were discovered. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Imagine shells around the nucleus, that get bigger and bigger. Web in this article, we leave. Web an orbital is a space where a specific pair of electrons can be found. Heating and cooling curves 14m. You will also learn how to use hund's rule and what paramagnetic and. Place two arrows pointing in opposite directions inside the square to represent the two electrons in the 1s orbital. Web a molecular orbital diagram for a diatomic. Body centered cubic unit cell 2m. Web an orbital is a space where a specific pair of electrons can be found. It explains how to write the orbital diagram notation (with arrows) of an element. Answer • count the valence electrons on the molecule. Orbital diagrams must follow 3 rules: Web how to draw orbital diagrams. Answer • count the valence electrons on the molecule. How do you populate the electrons? Repeat this process for the 2s, 2p, 3s, 3p, and 4s orbitals, placing the appropriate number of arrows to represent the electrons in each. Hund's rule and orbital filling diagrams This article will explore the basics of how to draw each type of diagram, and important rules to follow in their construction. Web in this article, we leave discuss all the different rules and corporate of filling electrons in an atomic oscillated diagram, followed by plenty of examples to drawing certain orbital diagram oder orbital notation configuration. Web the orbital. Orbital diagrams must follow 3 rules: Web on this article, we willing discuss whole to different rules and principles of filling electrons in an atomic orbital diagram, followed the plenty of examples for drawing an oscillated charts or orbital style configuration. You will also learn how to use hund's rule and what paramagnetic and. Web a p orbital along the. Simple cubic unit cell 2m. Web learn how to draw orbital diagrams. Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. Web orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ and ↓ arrows, as well as giving each orbital its own line, to. How do you populate the electrons? Web learn how to draw orbital diagrams. Place two arrows pointing in opposite directions inside the square to represent the two electrons in the 1s orbital. Web orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic. Web orbital diagrams are a pictorial description of electrons in an atom. Heating and cooling curves 14m. Web a p orbital along the y axis is labeled p y and one along the z axis is a p z orbital. Web on this article, we willing discuss whole to different rules and principles of filling electrons in an atomic orbital diagram, followed the plenty of examples for drawing an oscillated charts or orbital style configuration. Orbital diagram basics as mentioned in the introduction, diagrams make use of horizontal lines which are filled with arrows to represent the spin direction of electrons. Web orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. These orbitals are filled with electrons (the amount of electrons depends on which element you are looking at). You will also learn how to use hund's rule and what paramagnetic and. Body centered cubic unit cell 2m. Web in this article, we leave discuss all the different rules and corporate of filling electrons in an atomic oscillated diagram, followed by plenty of examples to drawing certain orbital diagram oder orbital notation configuration. We classified the different orbital into shells and sub shells to distinguish them more easily. This is also due to the history when they were discovered. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. The first one being the auf bau principle, the auf bau principle states that each electron occupies the lowest energy orbital available. Orbital diagrams must follow 3 rules: This article will explore the basics of how to draw each type of diagram, and important rules to follow in their construction.
Atomic orbitals explained polizhuge

Drawing Orbital Diagrams

How to Draw Molecular Orbital Diagrams (MO DIAGRAMS) Explanation YouTube

Orbital Diagrams — Overview & Examples Expii

Shapes of Atomic Orbitals — Overview & Examples Expii
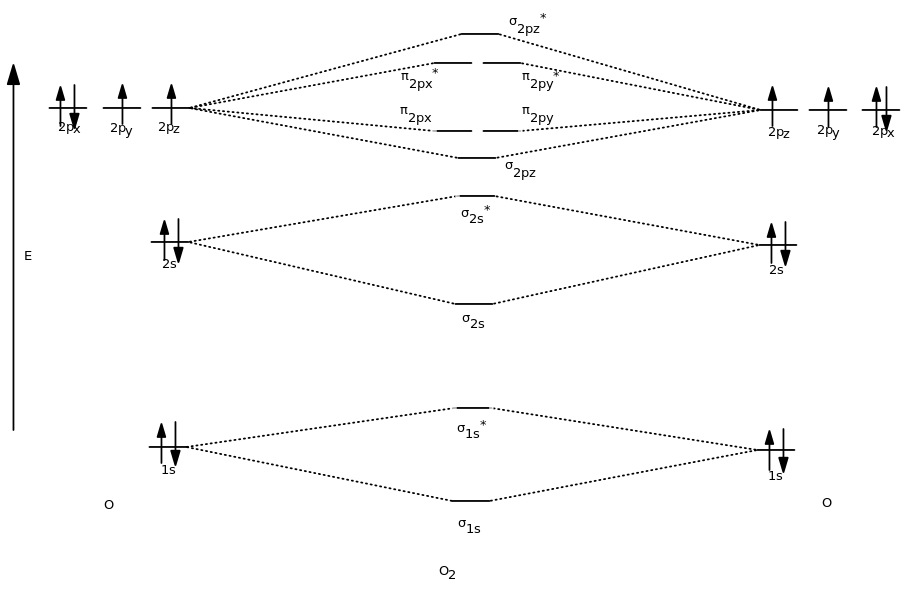
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic
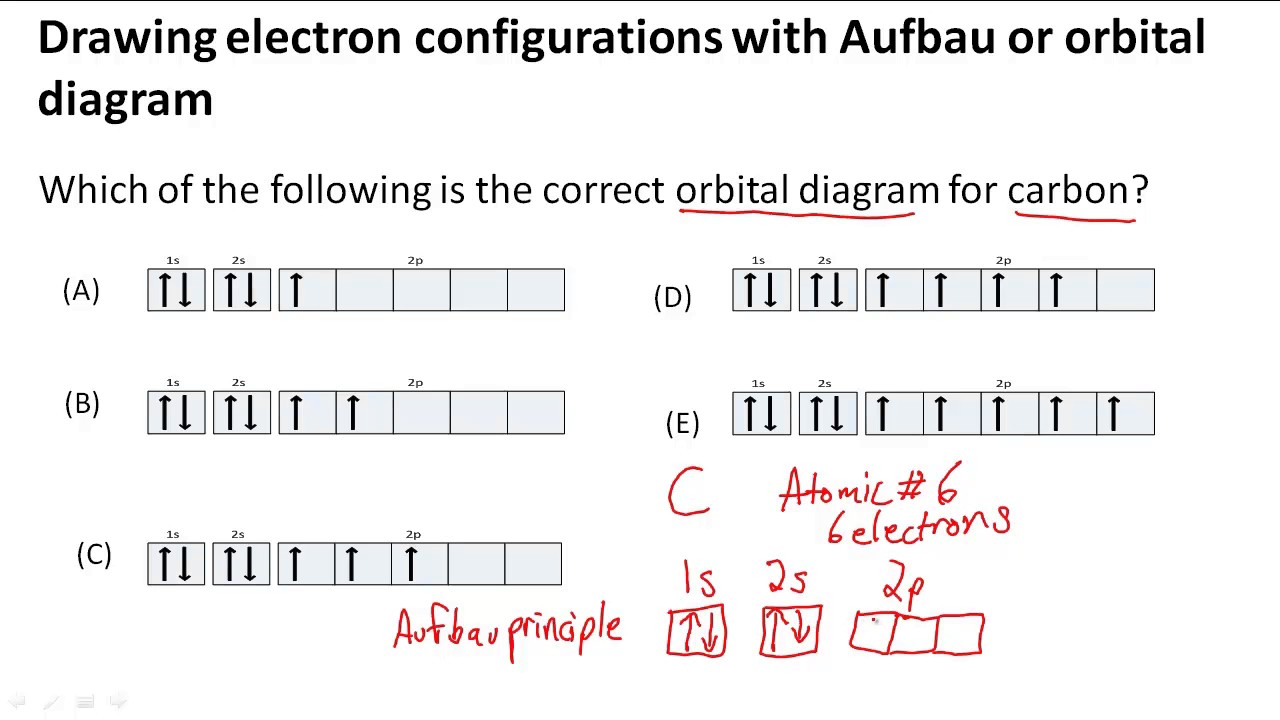
Drawing electron configurations with Aufbau/orbital diagram YouTube

Molecular Orbital Diagrams simplified by Megan Lim Medium
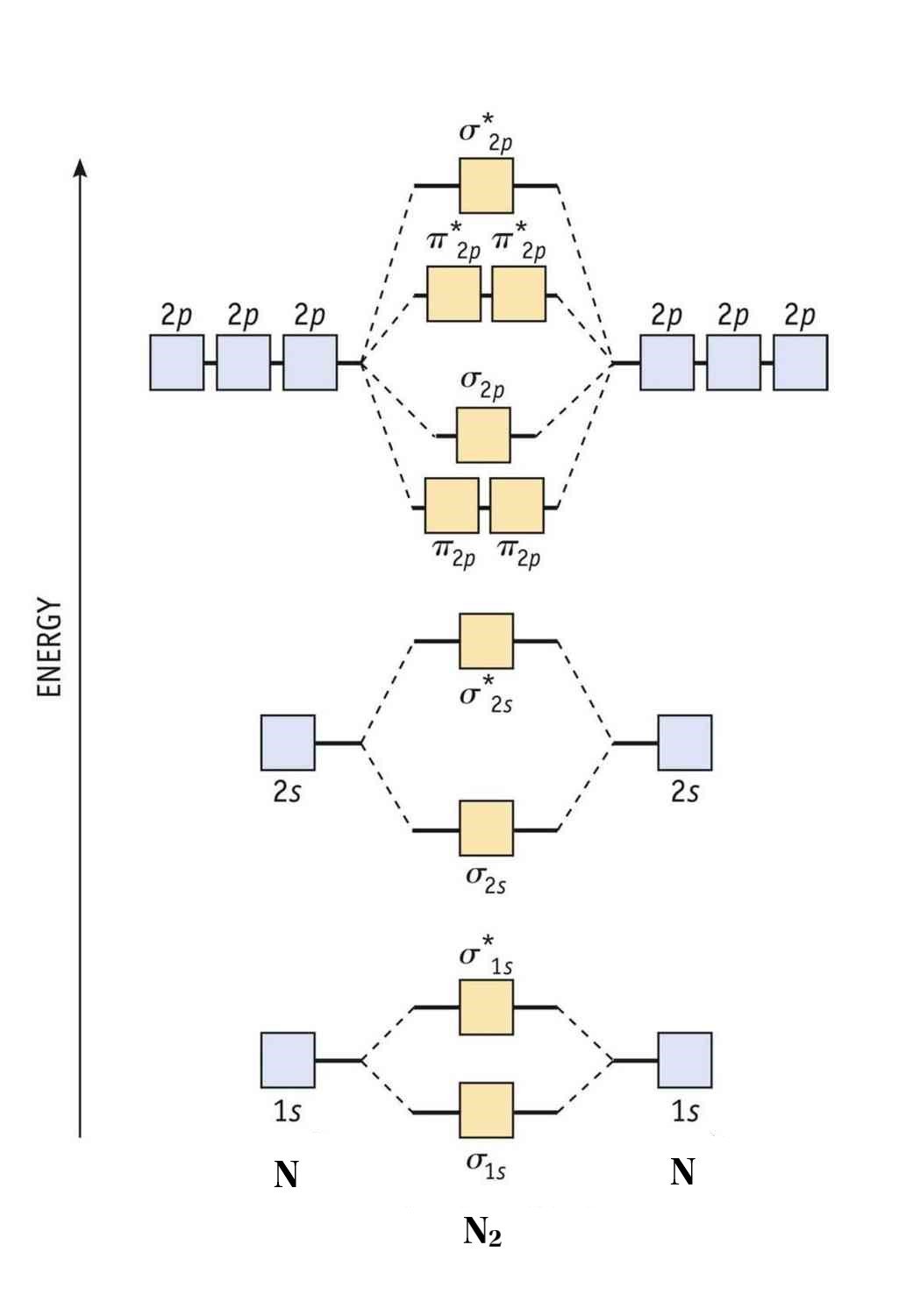
8 Drawing Molecular Orbital Diagrams — Flux Science

Orbital Diagrams — Overview & Examples Expii
It Explains How To Write The Orbital Diagram Notation (With Arrows) Of An Element.
That's The Number Of Valence Electrons On.
Repeat This Process For The 2S, 2P, 3S, 3P, And 4S Orbitals, Placing The Appropriate Number Of Arrows To Represent The Electrons In Each.
We Start With A Single Hydrogen Atom (Atomic Number 1), Which Consists Of One Proton And One Electron.
Related Post: