How To Draw The Atomic Structure
How To Draw The Atomic Structure - A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. For example, all hydrogen atoms have one proton. Each element has its own atomic number, which is equal to the number of protons in its nucleus. How to draw an atom | atom structure diagram | physics project diagram subscribe for more videos: Web it also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. The element atomic number and name are listed in the upper left. Web how to draw an atomic structure yokidz 344k subscribers subscribe 659 share 47k views 5 years ago science projects drawing | body organs | body parts let's learn how to draw an atomic. Atoms themselves are composed of protons, neutrons, and electrons. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. Web the basic structure of an atom consists of a nucleus containing protons and neutrons and a cloud of electrons that circle the nucleus. Web. In the bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons. Elements and atoms elements and atoms matter, elements, and atoms introduction to the atom atomic structure atomic number, atomic mass, and isotopes atomic structure google classroom what three particles make up an atom? Web it also depicts. Unit 1 welcome to physical chemistry. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. Advances in nuclear and subatomic physics. Quantum field theory and the standard model The laws of quantum mechanics; Unit 3 some basic concepts of chemistry. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. Chemistry is based on the modern atomic theory, which states that all matter is composed of atoms. Web faqs the advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. A. The element atomic number and name are listed in the upper left. The discovery of subatomic particles has been the base for many other discoveries and inventions. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web the basic structure of an atom consists of a nucleus containing protons. In the bohr model of the atom, the nucleus contains the majority of the mass of the atom in its protons and neutrons. Each element has its own atomic number, which is equal to the number of protons in its nucleus. It is the smallest of the main 3 particles that comprise an atom. The numbers of subatomic particles in. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic. Web it also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. The element atomic number and name are listed in the upper left. The number of protons. Web the basic structure of an atom consists of a nucleus containing protons and neutrons and a cloud of electrons that circle the nucleus. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Chemistry is based on the modern atomic theory, which states that all matter is composed. Web drawing atoms (ncea l1 & junior science) student note: This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from periodic. Elements and atoms elements and atoms matter, elements, and atoms introduction to the atom atomic structure atomic number, atomic mass, and isotopes atomic structure google classroom. The electron shells are shown, moving outward from the nucleus. Web atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. All atom labels are shown and all lone pairs are shown. How to draw an atom | atom structure diagram | physics project diagram subscribe for more videos: Web simple rules to guide you to. Unit 2 structure of atom. The electron shells are shown, moving outward from the nucleus. Web drawing atoms (ncea l1 & junior science) student note: It is the smallest of the main 3 particles that comprise an atom. Advances in nuclear and subatomic physics. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Covalent bonds are shown using lines. An electron is a negatively charged particle. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. The number of protons in the nucleus of an atom determines its atomic number and its identity as a specific element. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Atoms themselves are composed of protons, neutrons, and electrons. Antiparticles and the electron’s spin; Unit 3 some basic concepts of chemistry. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element.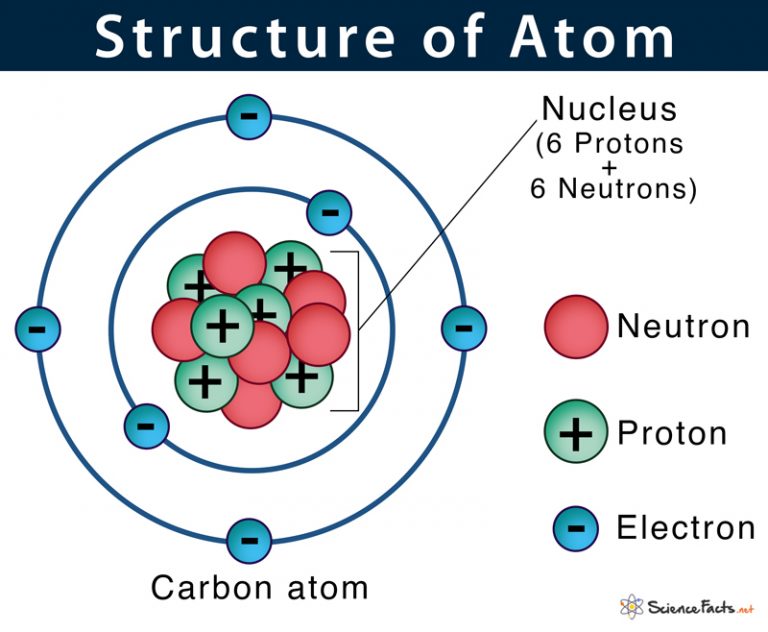
Atom Definition, Structure & Parts with Labeled Diagram

Drawing Atoms Montessori Muddle
How to Draw an Atom Step by Step Simple and Easy YouTube
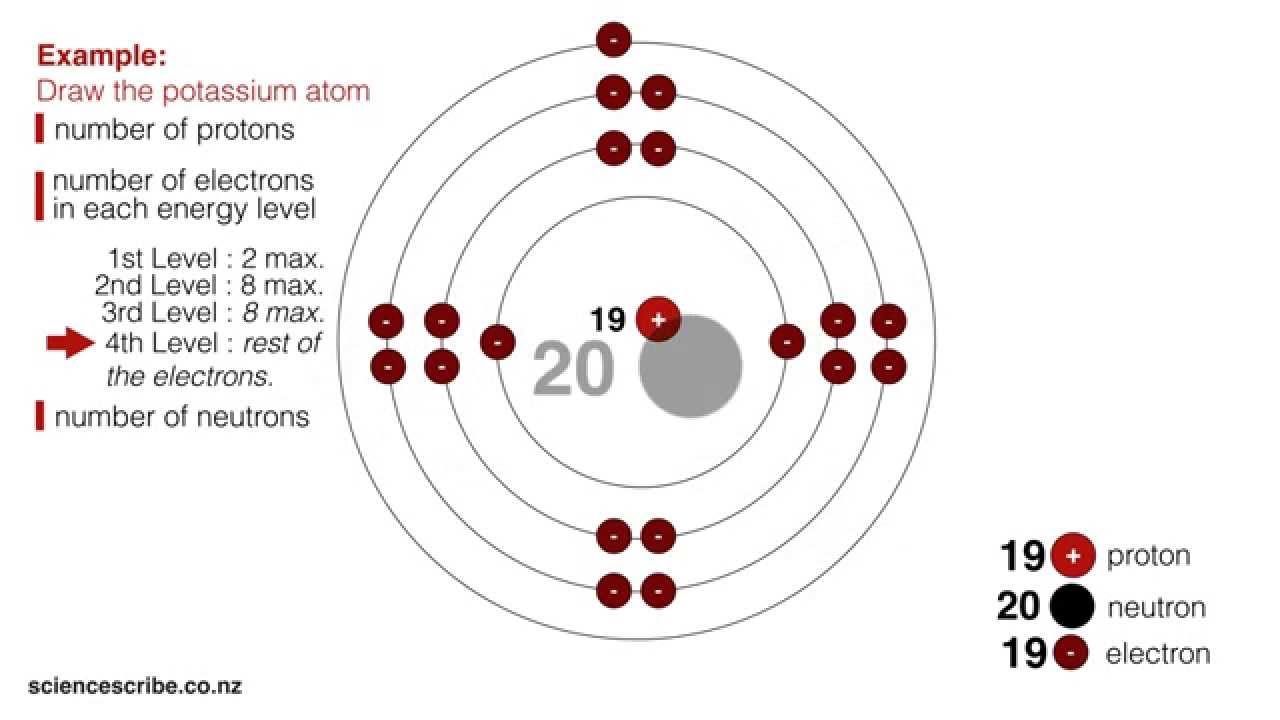
Drawing Atoms (NCEA L1 & Junior Science) YouTube
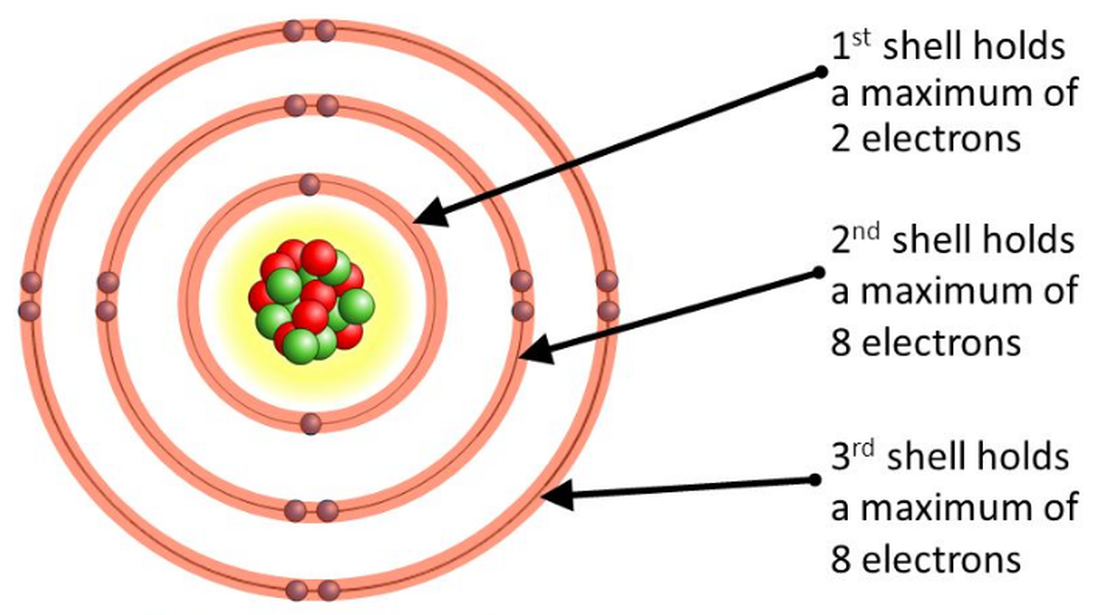
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

Atom Definition, Structure & Parts with Labeled Diagram

How to Draw an Atom Really Easy Drawing Tutorial
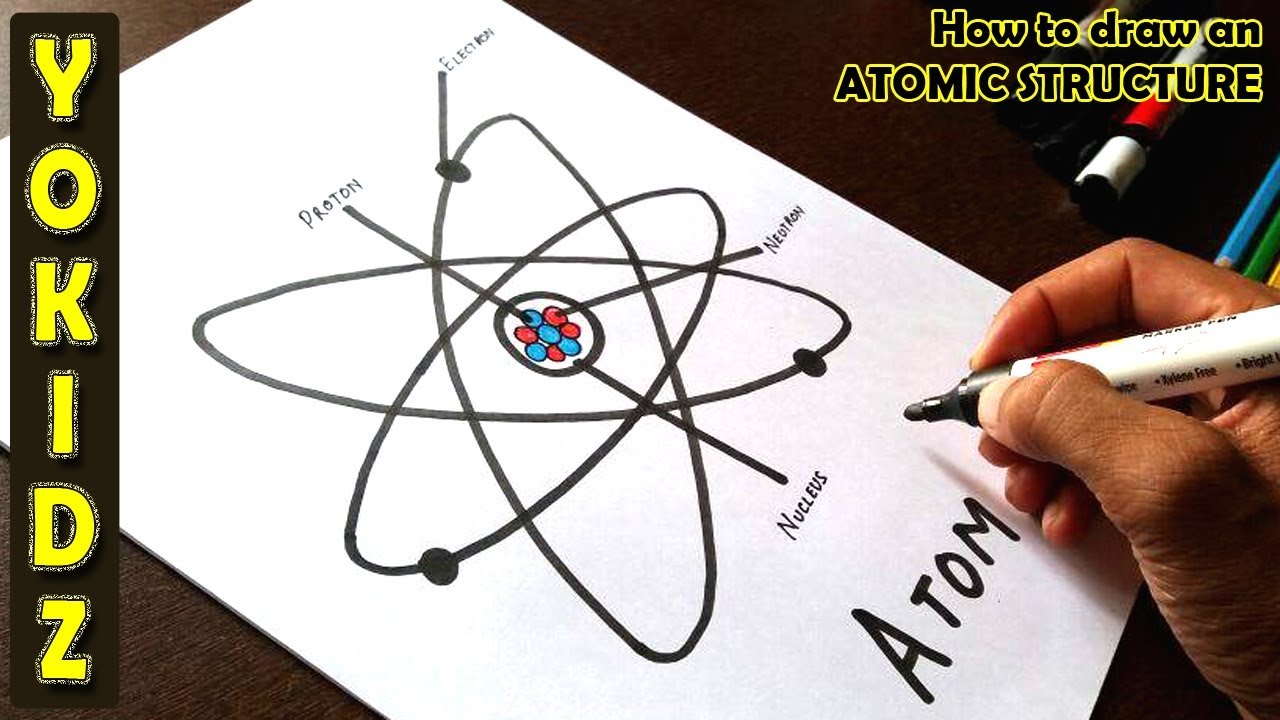
How to draw an ATOMIC structure YouTube

Draw A Simple Diagram Of An Atom Labeled Protons Neutrons And Electrons

Simple model of atom structure with electrons vector image on
Web Simple Rules To Guide You To Draw Atomic Structures!
The Discovery Of Subatomic Particles Has Been The Base For Many Other Discoveries And Inventions.
Each Element Has Its Own Atomic Number, Which Is Equal To The Number Of Protons In Its Nucleus.
Web Atoms Consist Of A Nucleus Containing Protons And Neutrons, Surrounded By Electrons In Shells.
Related Post: