Kenalog Dilution Chart
Kenalog Dilution Chart - You’ll find key information about kenalog below. For the purpose of comparison, the following is the equivalent milligram dosage of the various glucocorticoids: These dose relationships apply only to oral or intravenous administration of these compounds. Healthcare professionals (smpc) patient leaflet (pil) the patient information leaflet (pil) is the leaflet included in the pack with a medicine. It also allows the medication to be distributed to a wider area and may decrease the risk of local skin atrophy. Additionally, 50.5 percent of respondents counsel patients on potential adverse effects of hypopigmentation and atrophy before every injection. Sodium hydroxide or hydrochloric acid may have been added to adjust ph between 5.0 and 7.5. Web diluting the steroid in lidocaine may reduce the pain of injection. 160 mg im qday for 1 week, followed by 64 mg every other day for 1 month. This formulation is not for intradermal injection. Bristol myers squibb pharmaceuticals limited see contact details. Enter a drug name and triamcinolone acetonide injectable suspension. Web the most frequently used volume injected was 0.05ml (42.3%). It also allows the medication to be distributed to a wider area and may decrease the risk of local skin atrophy. Web the range of initial doses is 0.11 to 1.6 mg/kg/day in. Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose. Includes dose adjustments, warnings and precautions. You’ll find key information about kenalog below. It also allows the medication to be distributed to a wider area and may decrease the risk of local skin. A 20% final product dilution. Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose. You’ll find key information about kenalog below. 2.5 to 5 mg (0.063 to 0.125 ml) for smaller joints and from 5 to 15 mg (0.125 to 0.375 ml). Each ml of the sterile aqueous suspension provides 10 mg triamcinolone acetonide, with 0.66% sodium chloride for. Diluting the steroid and injecting the steroid into the correct location are the most crucial aspects of this process. Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a. Web the range of initial doses is 0.11 to 1.6 mg/kg/day in 3 or 4 divided doses (3.2 to 48 mg/m 2 /day). A 20% final product dilution. You’ll find key information about kenalog below. Web each ml of the sterile aqueous suspension provides 10 mg triamcinolone acetonide, with 0.66% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a. Additionally, 50.5 percent of respondents counsel patients on potential adverse effects of hypopigmentation and atrophy before every injection. Web an intralesional steroid injection involves a corticosteroid such as triamcinolone acetonide injected directly into a lesion on or immediately below the skin. Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99%. Single injections into several joints, up to a total of 20 mg or more, have been given. For the purpose of comparison, the following is the equivalent milligram dosage of the various glucocorticoids: Web each ml of the sterile aqueous suspension provides 10 mg triamcinolone acetonide, with 0.66% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.63%. These dose relationships apply only to oral or intravenous administration of these compounds. Web the most frequently used volume injected was 0.05ml (42.3%). Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose. Web the standard injectable steroid in dermatology is triamcinolone acetonide. This formulation is not for intradermal injection. Web an intralesional steroid injection involves a corticosteroid such as triamcinolone acetonide injected directly into a lesion on or immediately below the skin. Includes dose adjustments, warnings and precautions. Bristol myers squibb pharmaceuticals limited see contact details. Sodium hydroxide or hydrochloric acid may have been added to adjust ph between 5.0 and 7.5. 2.5 to 5 mg (0.063 to 0.125 ml) for smaller joints and from 5 to 15 mg (0.125 to 0.375 ml) for larger joints, depending on the specific disease entity being treated. You’ll find key information about kenalog below. Web each ml of the sterile aqueous suspension provides 10 mg triamcinolone acetonide, with 0.66% sodium chloride for isotonicity, 0.99% (w/v). Includes dose adjustments, warnings and precautions. A 20% final product dilution. Enter a drug name and triamcinolone acetonide injectable suspension. This formulation is not for intradermal injection. Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose. Web the range of initial doses is 0.11 to 1.6 mg/kg/day in 3 or 4 divided doses (3.2 to 48 mg/m 2 /day). For the purpose of comparison, the following is the equivalent milligram dosage of the various glucocorticoids: Additionally, 50.5 percent of respondents counsel patients on potential adverse effects of hypopigmentation and atrophy before every injection. Avoid injecting >0.1 ml or >10 mg/ml per site (3 ml total per session) For adults, doses up to 10 mg (0.25 ml) for smaller areas and up to 40 mg (1 ml) for larger areas have usually been sufficient. Includes dose adjustments, warnings and precautions. Diluting the steroid and injecting the steroid into the correct location are the most crucial aspects of this process. Inject 0.1 ml into patch at 1 cm intervals; Web each ml of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, with sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose. Web dilute kenalog 40 mg/ml with saline to 10 mg/ml. Single injections into several joints, up to a total of 20 mg or more, have been given.
Kenalog10 FDA prescribing information, side effects and uses

KENALOG40 (Dispensing Solutions, Inc.) FDA Package Insert

KENALOG10 (Dispensing Solutions, Inc.) FDA Package Insert
Kenalog40 Uses, Dosage, Side Effects, Warnings
Kenalog Inj Vial 40mg/ml 6 X 1 Ml Nonreturnable « Medical Mart

Kenalog40 40mg/mL Injection 10mL/Vial Modern Medical Products

Dilution Guidelines
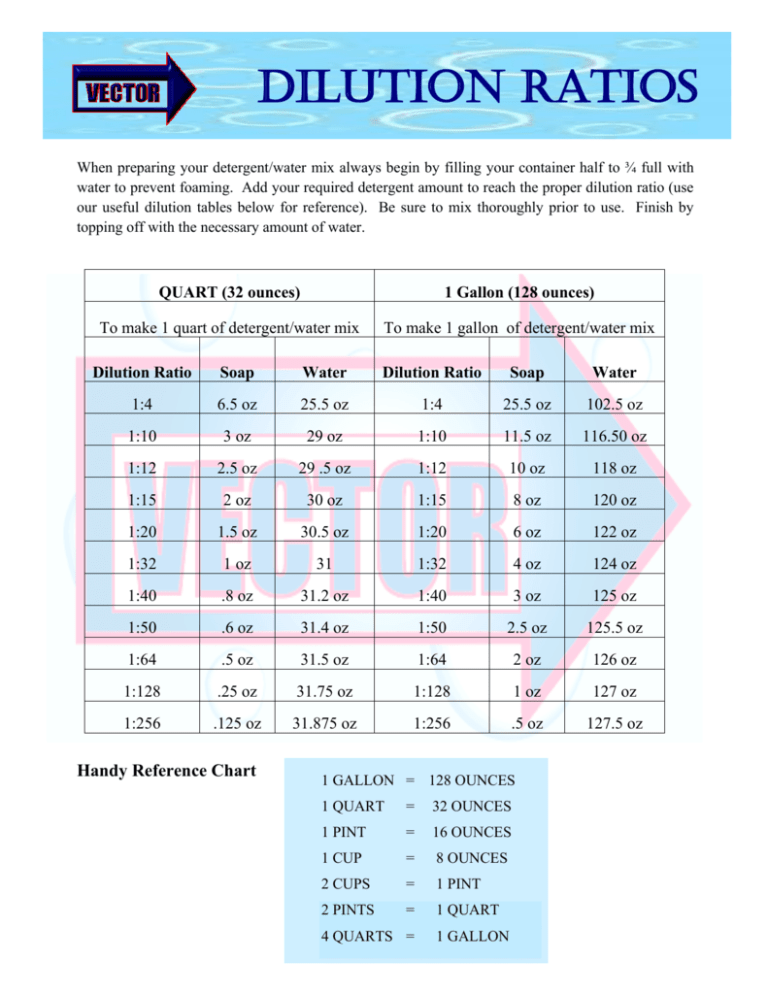
Dilution Ratios Table

Chemical Dilution Chart

Kenalog 40mg/ml Injection (5x1ml) Teleta Pharma
Web The Standard Injectable Steroid In Dermatology Is Triamcinolone Acetonide (Kenalog).
Web Each Ml Of The Sterile Aqueous Suspension Provides 10 Mg Triamcinolone Acetonide, With 0.66% Sodium Chloride For Isotonicity, 0.99% (W/V) Benzyl Alcohol As A Preservative, 0.63% Carboxymethylcellulose Sodium, And 0.04% Polysorbate 80.
Healthcare Professionals (Smpc) Patient Leaflet (Pil) The Patient Information Leaflet (Pil) Is The Leaflet Included In The Pack With A Medicine.
These Dose Relationships Apply Only To Oral Or Intravenous Administration Of These Compounds.
Related Post: