Metal Reactivity Series Chart
Metal Reactivity Series Chart - Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) Of metals is a chart showing metals in order of decreasing. Web metals can be ordered according to their reactivities; An easy way to use the table is to note that a metal can reduce any ion of a metal below it in the table. The top metals are more reactive than the metals on the bottom. The table below is an activity series of most common metals and of. So, the reactivity series of metals can be defined as a series of metals, in order of reactivity from highest to lowest. The more vigorously it reacts with other. Similarly, if a metal is below hydrogen in the reactivity series, then it cannot displace hydrogen from water and acids, and thus, will not produce hydrogen gas upon reaction with water and acids. Web below is a chart showing the reactivity series of common metals: Web reactivity series of metals, also referred to as the activity series, is the arrangement of metals in the descending order of their activities. A metal listed above another metal will replace that metal in a single replacement (single displacement) reaction. Web the reactivity series of metals is a chart listing metals in order of decreasing reactivity. The top metals. Aluminium is protected from contact with water by a natural layer of aluminium oxide. Web the reactivity series is a series of metals, in order of reactivity from highest to lowest. The top metals are more reactive than the metals on the bottom. Web the activity series is a chart of metals listed in order of declining relative reactivity. A. Web the activity series is a list of elements in decreasing order of their reactivity. Metals tend to readily lose electrons and form cations. Web below is a chart showing the reactivity series of common metals: A more reactive metal will displace a less reactive metal from a compound. The tables show how the elements react with water and dilute. Web reactivity series is the series of metals based on their reactivity from highest to lowest. The top metals are more reactive than the metals on the bottom. Web in chemistry, a reactivity series (or reactivity series of elements) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their reactivity from highest to lowest.. Most of them react with atmospheric oxygen to form metal oxides. If a reaction would occur then complete the word equation and balanced symbol equations. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. At the bottom are the least reactive metals. The more vigorous its reactions are. A metal listed above another metal will replace that metal in a single replacement (single displacement) reaction. The table below shows a selection of common metals and their reactivities with water, air, and dilute acids. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. The table \(\pageindex{1}\) below. A more reactive metal will displace a less reactive metal from a compound. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. Of metals is a chart showing metals in order of decreasing. Web the activity series is a list of elements in decreasing order of their reactivity.. The more vigorously it reacts with other. Observations of the way that these elements react with water, acids and steam enable us to put them into this series. The table below shows a selection of common metals and their reactivities with water, air, and dilute acids. Of metals is a chart showing metals in order of decreasing. At the top. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Web metals like potassium, sodium, calcium, magnesium, aluminium, zinc, iron, tin and lead are more reactive than hydrogen. The table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the. Web the activity. Most of them react with atmospheric oxygen to form metal oxides. Observations of the way that these elements react with water, acids and steam enable us to put them into this series. Metal activity or reactivity series finds its utility in the study of displacement reactions. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a. Web a chart of the reactivity series of common metals is provided below. An easy way to use the table is to note that a metal can reduce any ion of a metal below it in the table. Web metals can be ordered according to their reactivities; These metals can get tarnished or corrode very easily. Web below is a chart showing the reactivity series of common metals: Of metals is a chart showing metals in order of decreasing. Mg (s) + 2 h + (aq) → h 2 (g) + mg 2+ (aq) For example, both magnesium and zinc can react with hydrogen ions to displace h 2 from a solution by the reactions: Web the reactivity series is a hierarchical arrangement of elements based on their relative tendency to undergo chemical reactions. At the bottom are the least reactive metals. Web metal reactivity series is a list in which metals are arranged in the decreasing order of their chemical activity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Metal activity or reactivity series finds its utility in the study of displacement reactions. Web the reactivity series is a series of metals, in order of reactivity from highest to lowest. In general, the more reactive a metal is: The table below is an activity series of most common metals and of.
CSEC Chemistry Reactivity of Metals
![Reactivity Series of Metals Chart [and How to remember] Teachoo](https://d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/b0a28f61-1fb3-456e-8ae8-e110e8999f99/reactivity-series-01.jpg)
Reactivity Series of Metals Chart [and How to remember] Teachoo

The Reactivity Series Revision Notes in GCSE Chemistry
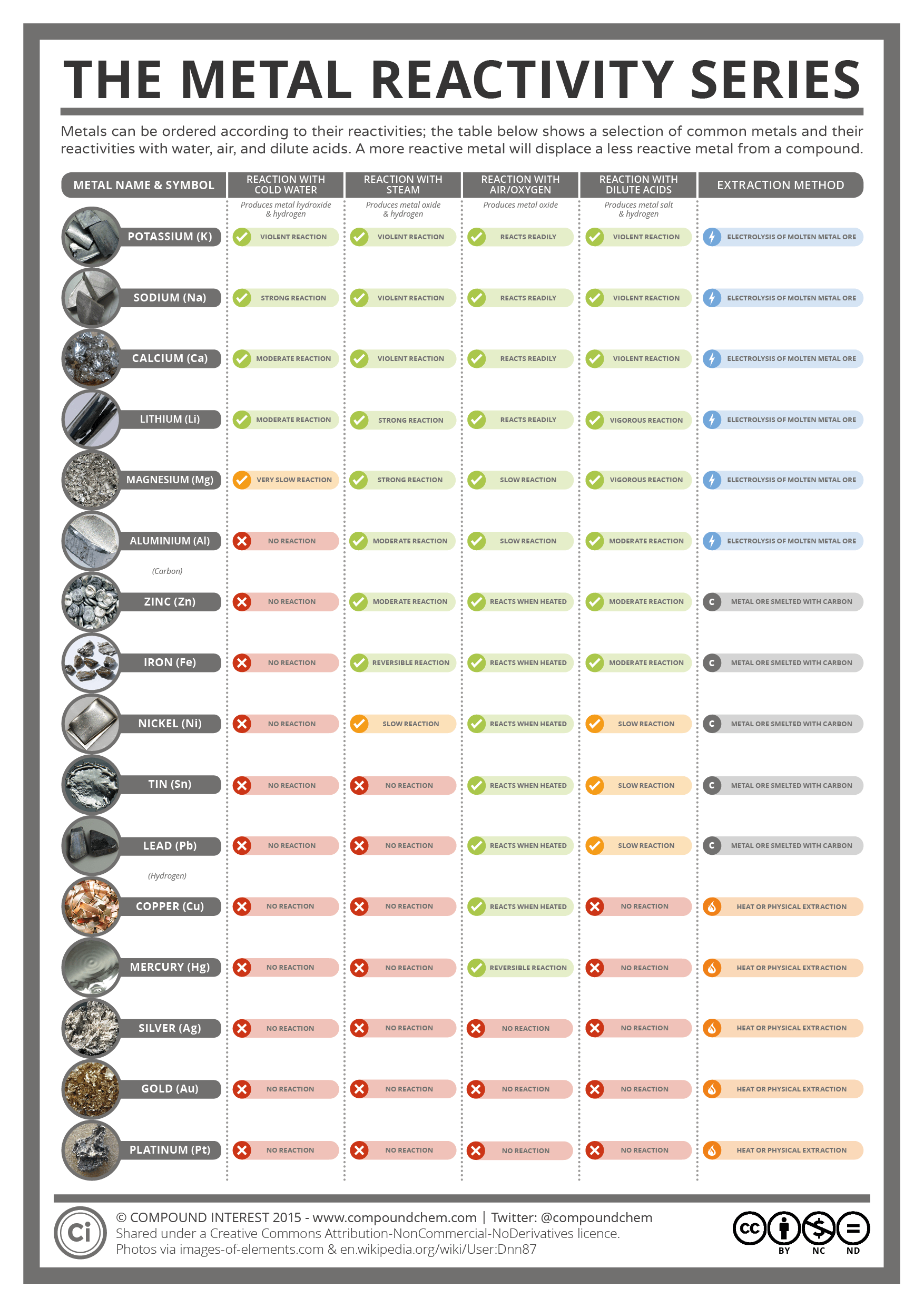
The Metal Reactivity Series Compound Interest
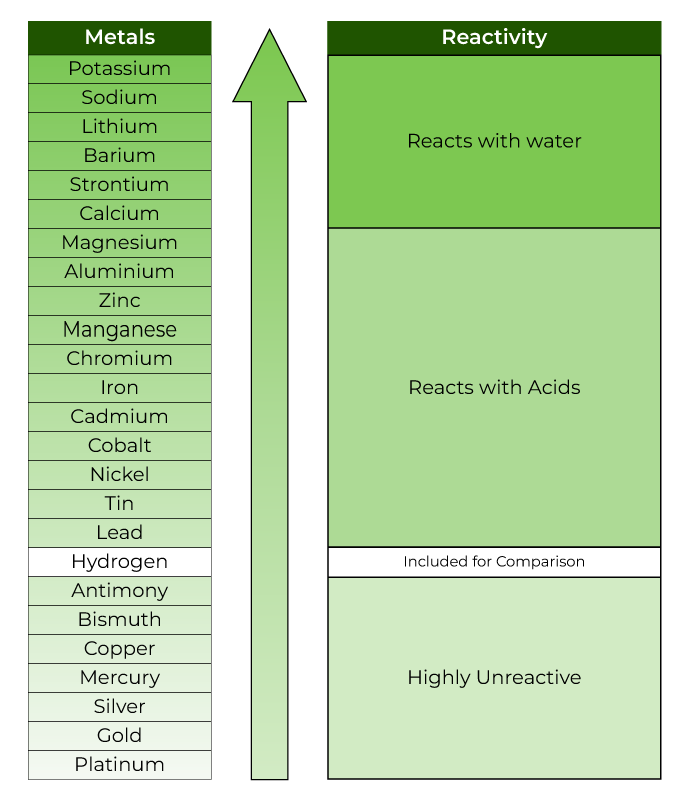
Reactivity Series Reactivity Of Metals Chart Features Uses

Activity Series of Metals (Reactivity Series)
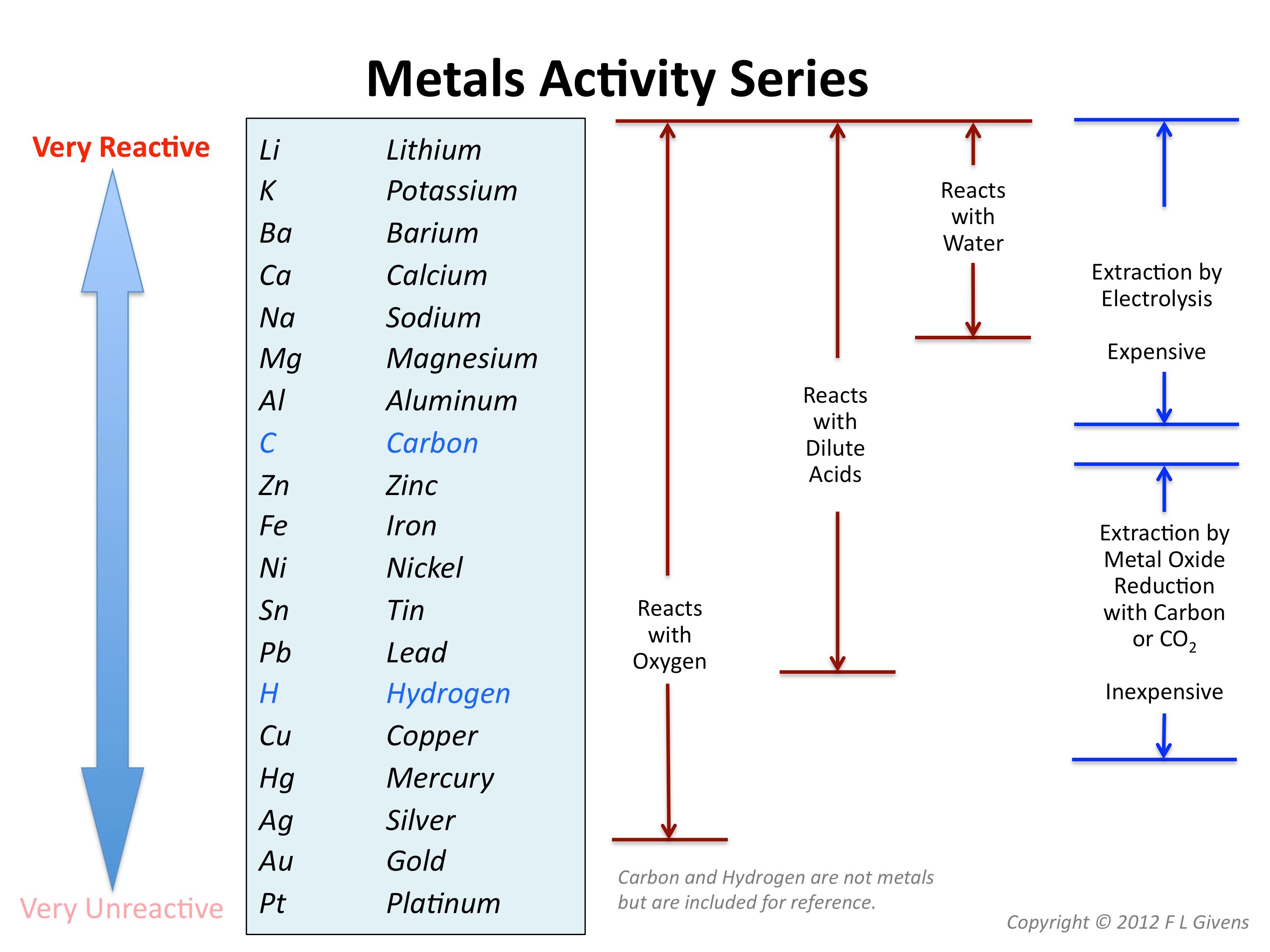
C10 Metals Science) Mr Cartlidge's second Science Blog
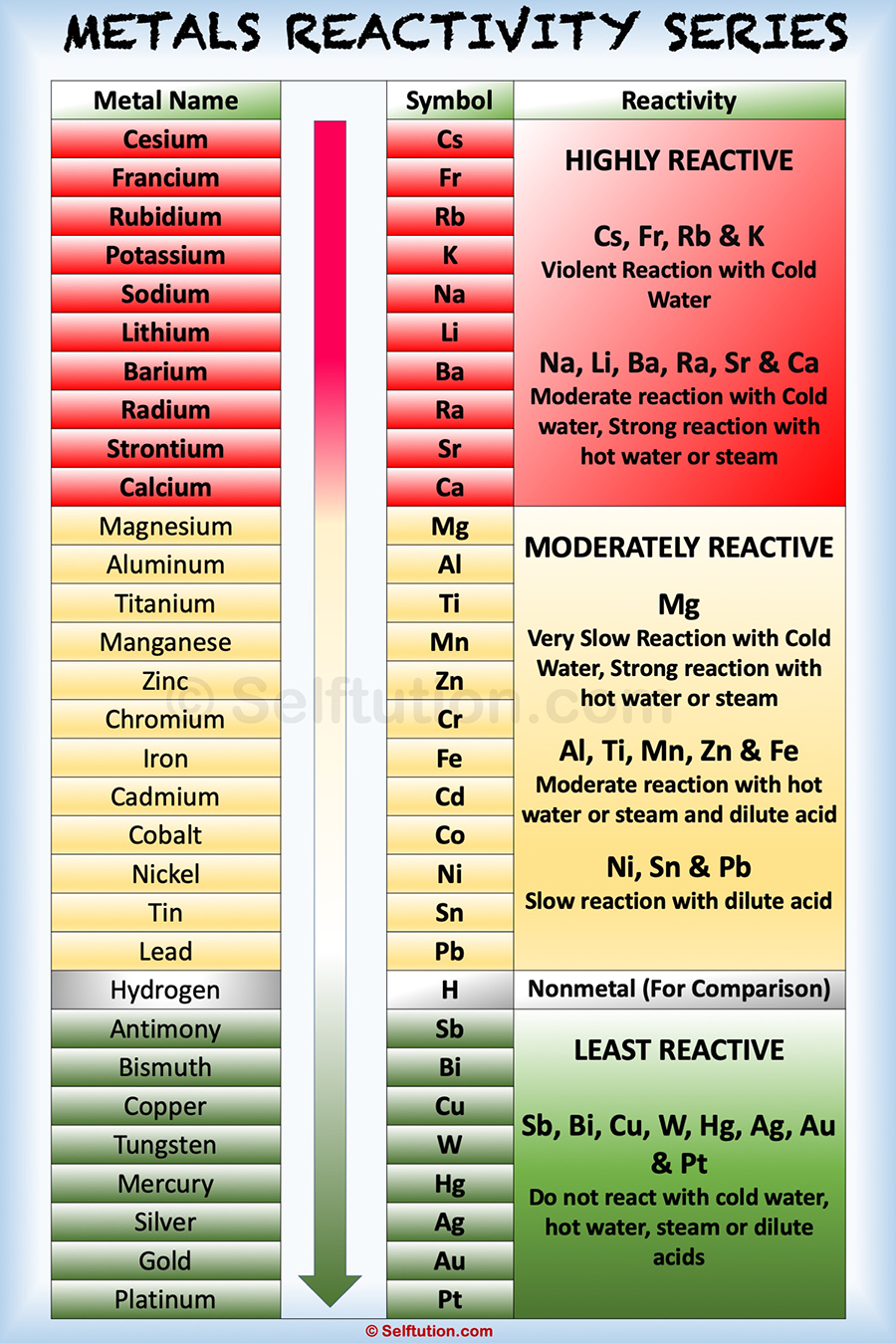
Reactivity Series of Metals and Nonmetals » Selftution
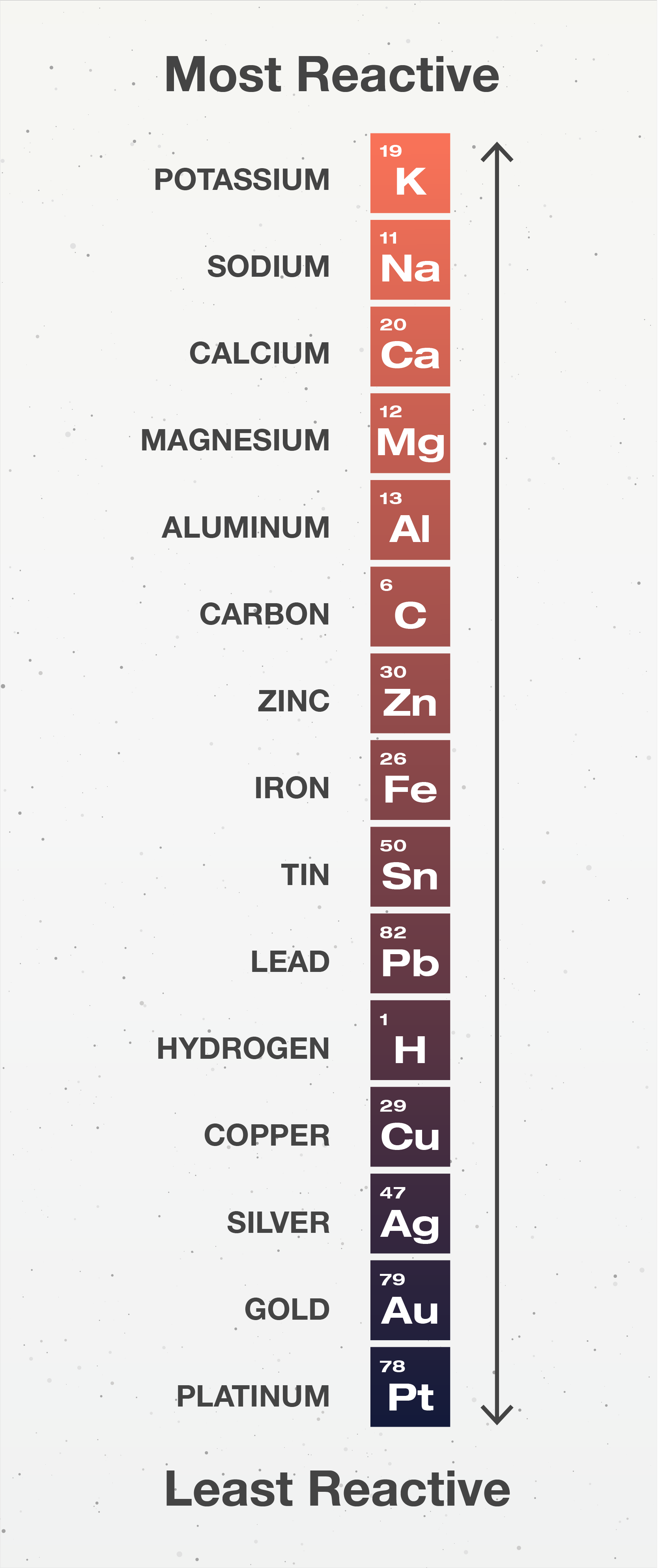
Metals Free Exam Academy
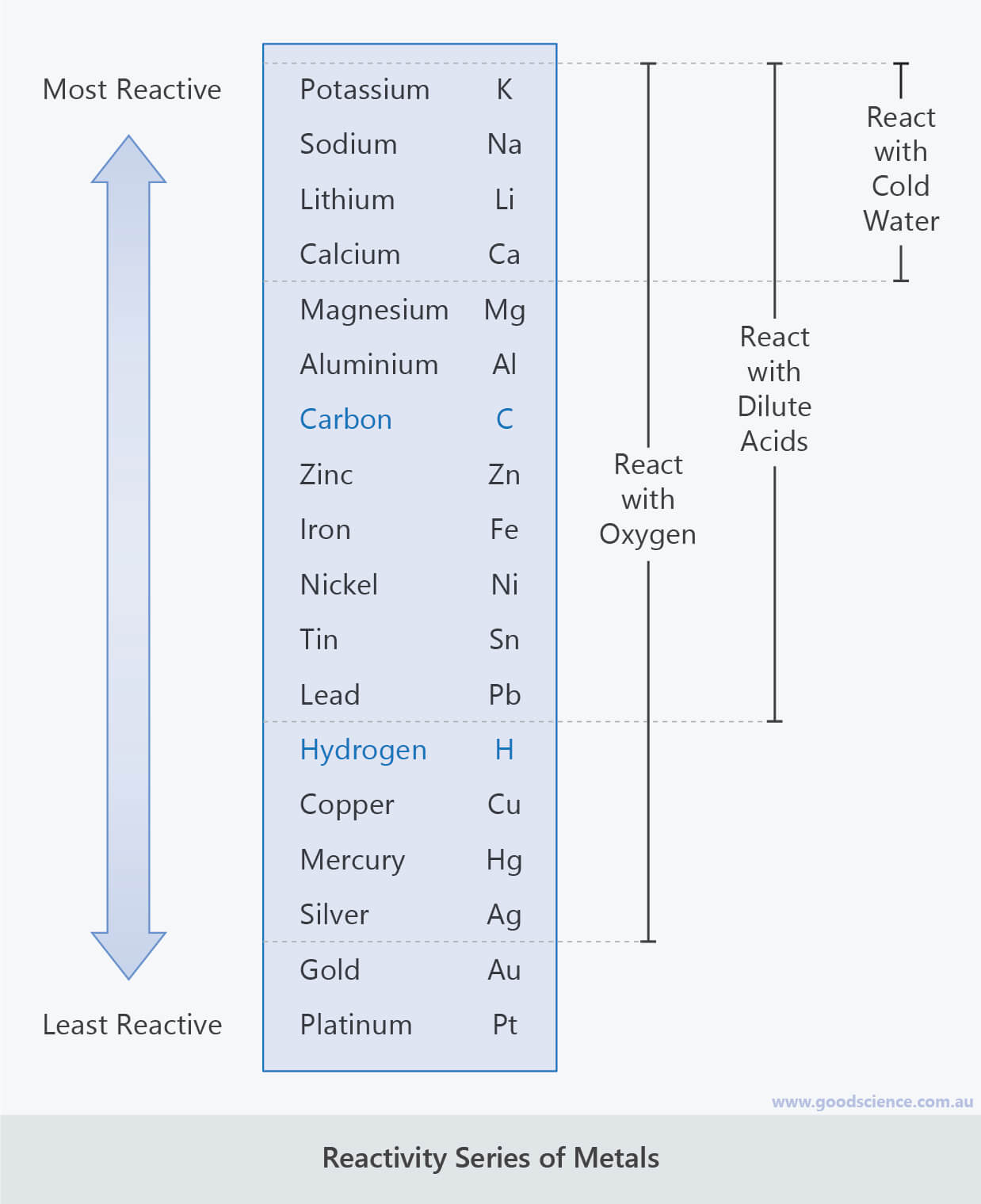
Metal Reactions Good Science
Web Reactivity Series Of Metals, Also Referred To As The Activity Series, Is The Arrangement Of Metals In The Descending Order Of Their Activities.
A More Reactive Metal Will Displace A Less Reactive Metal From A Compound.
The Table Below Shows A Selection Of Common Metals And Their Reactivities With Water, Air, And Dilute Acids.
Web This Graphic Places A Selection Of Common Metals Into Order Of Reactivity, As Well As Showing Their Reactions With Air, Water And Steam.
Related Post: