Practice Drawing Ionic Bonds
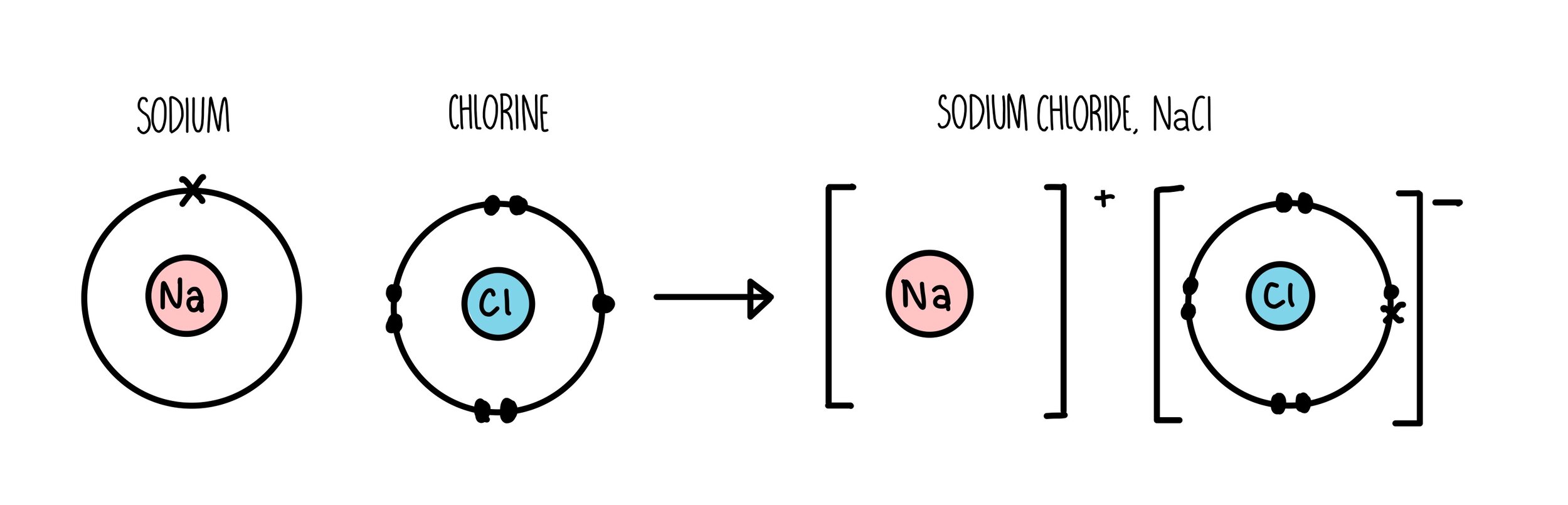
Practice Drawing Ionic Bonds - (a) ca(h 2 po 4) 2 (b) feso 4 (c) caco 3 (d) mgo (e) nano 2 (f) ki. Model1 substances are called ionic compounds and 2 substances are called covalent molecules. The skeletal structure of ethanethiol is shown below. This online quiz is intended to give you extra practice in identifying and drawing lewis dot structures as well as predicting ion formation this quiz aligns with the following ngss standard (s): A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Web ions of opposite charges can come together to form ionic bonds. There are 2 atoms of hydrogen (h) in the. Group #of valence electrons element #of protons oxidation ion that will form number add here! Periodic table ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. The astute reader may have noticed something: Web ionic bonding is made easy using interlocking shapes to represent cations and anions. Many of the ions that form have eight electrons in their valence shell. Sometimes not all of the ions dissolve. Periodic table ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. A dot and cross diagram is one. Write the chemical formula for each compound. Exercises what is the formula mass for the ionic compound formed by each pair of ions? Nonmetals) predicting bond type (electronegativity) electronegativity Write a simple rule that will allow you to classify compounds as ionic or covalent on the basis of what you have learned from the model. This tells you the #. (a) ca(h 2 po 4) 2 (b) feso 4 (c) caco 3 (d) mgo (e) nano 2 (f) ki. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Draw a lewis dot structure for the valence shell of each element. Web science chemistry library unit 9: These bonds are very. Web the following ionic compounds are found in common household products. Web google classroom you might need: Web practice packet unit 6: Be sure to include the charges. Web for each of the following ionic bonds: Then answer the questions that follow. Bonding 5 www.mrpalermo.com tying it together: Lithium #3 #3 23 sodium #3 23 23 23 add here! Many of the ions that form have eight electrons in their valence shell. For each ionic compound, you have been given the element names and the chemical formula. Drawing lonic bonds part 1: Web for each of the following ionic bonds: Aluminum 23 23 23 23 add here! This tells you the # of atoms present for the element and is located to the right of the # in a formula unit. Select your preferences below and click 'start' to give it a try! Write a simple rule that will allow you to classify compounds as ionic or covalent on the basis of what you have learned from the model. Name each of the compounds: Then answer the questions that follow. Web practice packet unit 6: Select your preferences below and click 'start' to give it a try! Web lewis dot structures quiz. Use the periodie table to fill in the chart below. \text {na} and \text {cl} form \text {nacl}, an ionic salt.) For each ionic compound, you have been given the element names and the chemical formula. Beryllium 23 #3 23 23 add here! Lithium #3 #3 23 sodium #3 23 23 23 add here! Web lewis dot structures quiz. Web lewis structures are mostly applied to covalent molecules, and while it is exceedingly uncommon you should do the same for ionic compounds. Nonmetals) predicting bond type (electronegativity) electronegativity Then answer the questions that follow. The astute reader may have noticed something: Web ions of opposite charges can come together to form ionic bonds. Chemistry library > unit 1 lesson 3: First, draw the electron dot diagrams for each element below the name. Beryllium 23 #3 23 23 add here! Web draw lewis structures for ionic compounds. Finding the formula of an ionic compound predict the charge on monatomic ions naming ionic compounds Group #of valence electrons element #of protons oxidation ion that will form number add here! In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. These bonds are very strong and as such the resulting ionic compounds often have very high melting points. Nonmetals) predicting bond type (electronegativity) electronegativity Browse videos, articles, and exercises by topic. Chemistry library > unit 1 lesson 3: Lithium #3 #3 23 sodium #3 23 23 23 add here! Bonding 5 www.mrpalermo.com tying it together: Web ions of opposite charges can come together to form ionic bonds. Second, draw the ionic bonding diagram for the compound. Web follow your teacher’s directions to complete each ionic bond. Draw a cartoon of an ionic compound that partially dissolves in water. Exercises what is the formula mass for the ionic compound formed by each pair of ions? Chemical bonds about this unit this unit is part of the chemistry library.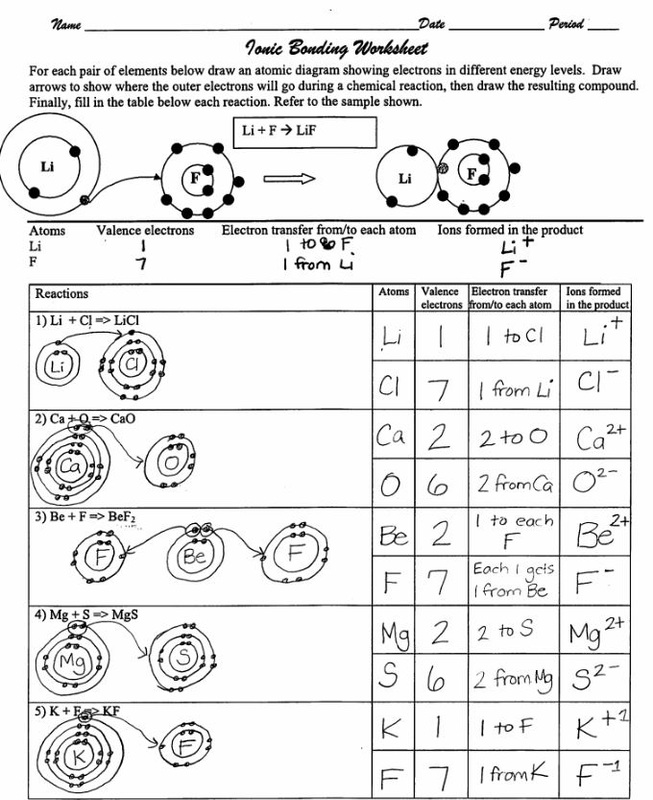
Ionic BOnding Diagrams 8th Grade Physical Science
Ionic Bonding — the science hive

Ionic Bond Definition, Types, Properties & Examples
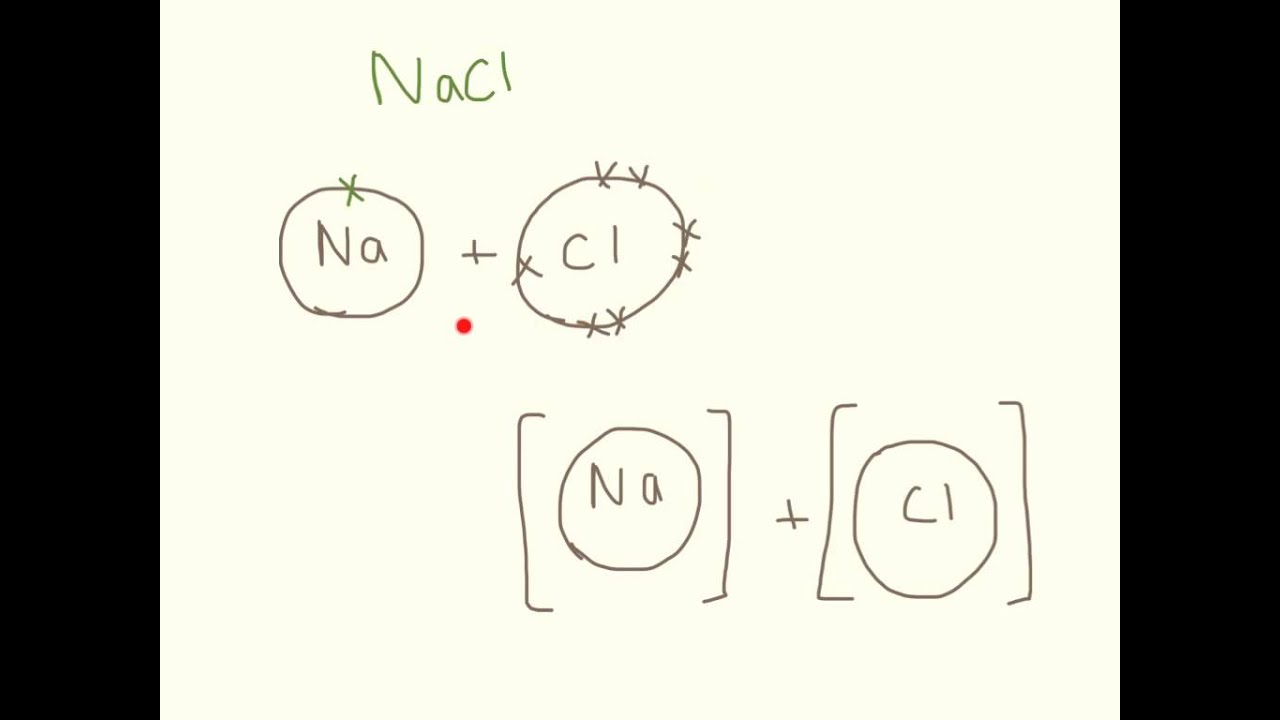
Drawing Ionic Bonding Dot and Cross Diagrams. YouTube
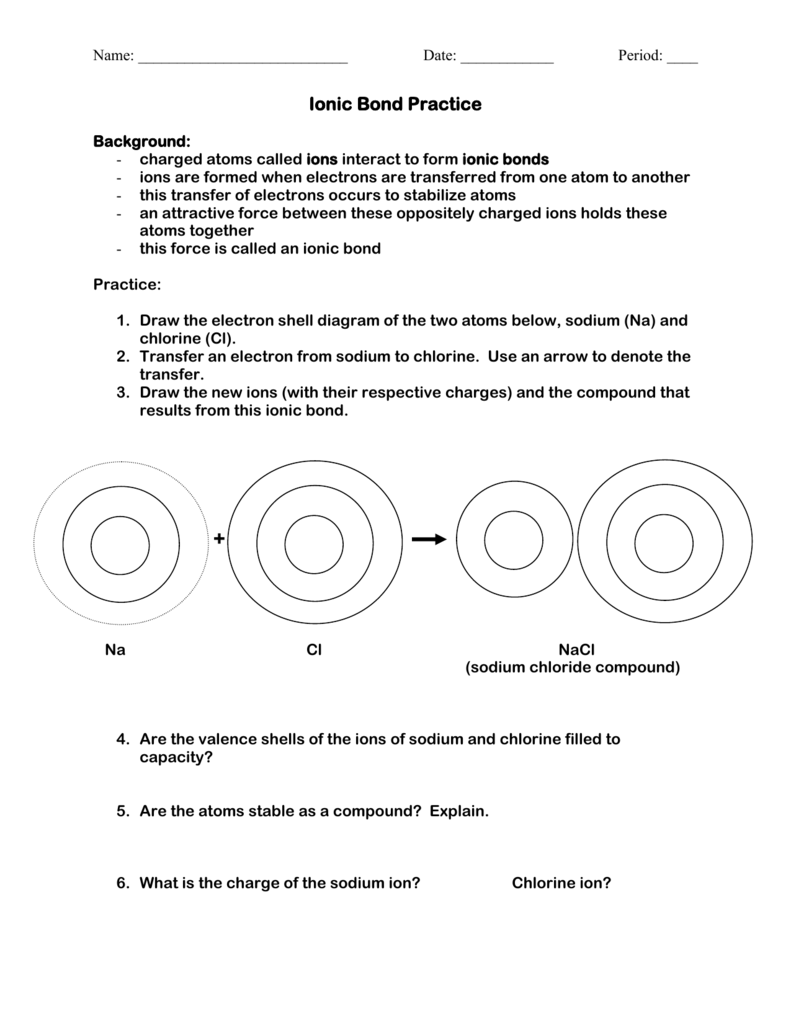
Ionic Bond Practice sciencewithskinner

Ionic And Covalent Bond Practice —
Ionic Bonding Practice Worksheet Answers Coearth

Chemistry Ionic Bonds Worksheet

Ionic Bonding Worksheet Chemistry

Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk
This Is A Combination Of Symbols That Shows The Ratio Of Elements In A Compound.
Use The Periodie Table To Fill In The Chart Below.
Sodium Is A Group 1 Metal So Will Lose One Outer Electron To Another Atom To Gain A Full Outer Shell Of Electrons.
There Are 2 Atoms Of Hydrogen (H) In The.
Related Post:
