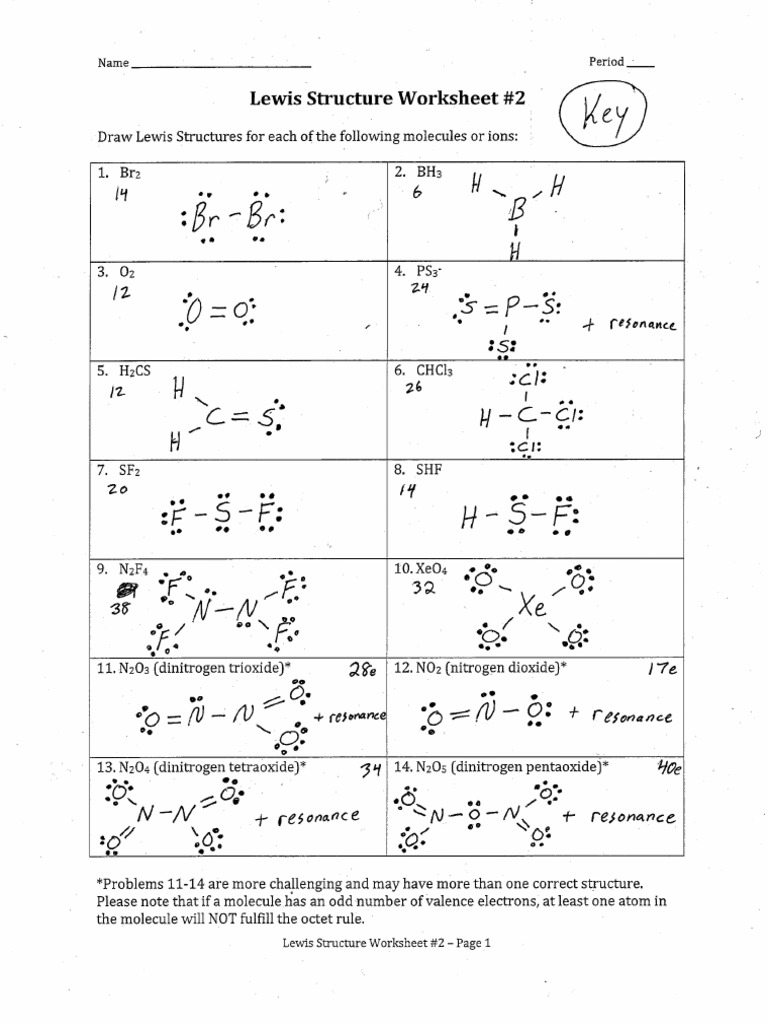
Practice Drawing Lewis Structures
Practice Drawing Lewis Structures - Web draw the best lewis dot structure for each of the following species. The video covers the basic lewis structures for a general chemistry class. Web every chemistry student has to learn how to draw lewis dot structures. Then name their electron arrangement, shape, and bond angles. Write the lewis structures for. In other cases choose the element that is the least electronegative (farthest to the left) on the periodic table. Web draw the lewis dot structure for each of the following polyatomic ions: Web drawing lewis diagrams google classroom about transcript a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The video covers the basic lewis structures you'll see in an introductory chemistry class. In these practice problems, we will work on determining the lewis structures of molecules and ions. Determine the shape of the molecule. The key is to understand the steps and practice. Web a video tutorial for how to draw lewis structures in five steps. Lewis diagram of formaldehyde (ch₂o) worked example: Draw the best lewis dot structures for each of the following species. Not even under a complex microscopic can we view the individual electrons that surround an atom’s nuclei. How the molecule might react with other molecules. The video covers the basic lewis structures for a general chemistry class. Web to practice drawing lewis structures for various covalently bonded molecules and polyatomic ions. Draw the lewis structures of the following polyatomic ions. Step 3) add electrons to all outer atoms. Lewis diagram of formaldehyde (ch₂o) worked example: Web draw the lewis structure for the following molecules. In these practice problems, we will work on determining the lewis structures of molecules and ions. A) bef 2 b) bcl 3 c) ccl 4 d) pbr 5 e) si 6. Carbon (c) will always be the central atom and hydrogen (h) will never be the central atom 2. Web a lewis structure is a graphic representation of the electron distribution around atoms. Shared pairs of electrons are drawn as lines between atoms, while. Not even under a complex microscopic can we view the individual electrons that surround an atom’s nuclei.. For the following molecules or ions (where the central atom is underlined): The physical properties of the molecule (like boiling point, surface tension, etc.). As a result, they must be equally satisfactory representations of the molecule. Web draw lewis structures for the following molecular formulas: Write the correct skeletal structure for the molecule. Determine if the molecule is polar or nonpolar. Web every chemistry student has to learn how to draw lewis dot structures. Lewis diagram of xenon difluoride (xef₂) lewis diagrams. If you are not sure if your structure is correct, do a formal charge check. Web a lewis structure is a graphic representation of the electron distribution around atoms. Web practice drawing lewis structures with answers and explanation. Web draw lewis structures for the following molecular formulas: Web concepts of chemical bonding. Web this chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2, o2, of2, ch4, nh3, h2o, c2h2, and n2h4. Web to practice drawing lewis structures for various covalently. Web practice drawing lewis structures with answers and explanation. The video covers the basic lewis structures you'll see in an introductory chemistry class. Determine the shape of the molecule. Write the lewis structures for. Two lewis structures can be written for sulfur dioxide. Web a lewis structure is a graphic representation of the electron distribution around atoms. Draw the lewis structures of the following polyatomic ions so2−3 so 3 2 − po3−4 po 4 3 − no−2 no 2 − pf+4 pf 4 + alcl−4 alcl 4 − answer practise drawing the lewis structure of molecules using the exercises below. The final answer. Draw the lewis structures of the following polyatomic ions so2−3 so 3 2 − po3−4 po 4 3 − no−2 no 2 − pf+4 pf 4 + alcl−4 alcl 4 − answer practise drawing the lewis structure of molecules using the exercises below. Web concepts of chemical bonding. The reason for learning to draw lewis structures is to predict the. A) bef 2 b) bcl 3 c) ccl 4 d) pbr 5 e) si 6. If you are not sure if your structure is correct, do a formal charge check. Two lewis structures can be written for sulfur dioxide. For the following molecules or ions (where the central atom is underlined): Web the arrangement of atoms in several biologically important molecules is given here. Web drawing lewis diagrams google classroom about transcript a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Lewis diagram of formaldehyde (ch₂o) worked example: In short, remember these steps for determining the lewis structure: Web draw the lewis dot structure for each of the following polyatomic ions: Draw the lewis structures of the following polyatomic ions so2−3 so 3 2 − po3−4 po 4 3 − no−2 no 2 − pf+4 pf 4 + alcl−4 alcl 4 − answer practise drawing the lewis structure of molecules using the exercises below. The key is to understand the steps and practice. Step 3) add electrons to all outer atoms. In these practice problems, we will work on determining the lewis structures of molecules and ions. Web a lewis structure is a graphic representation of the electron distribution around atoms. How the molecule might react with other molecules. The shape of a molecule.ChemE Lewis Structure Worksheet 2 Answers PDF
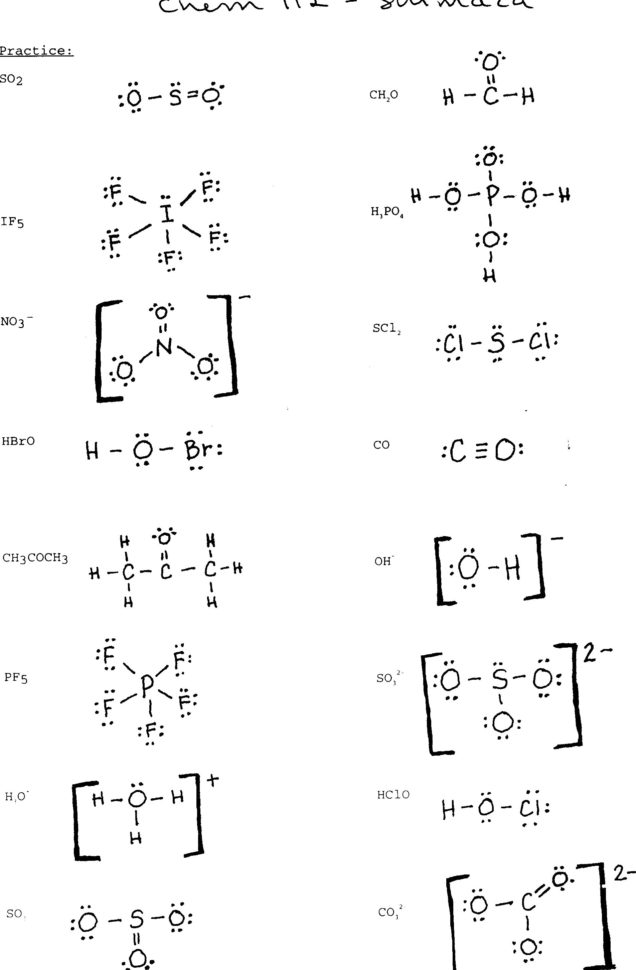
Lewis Dot Structure Practice Worksheet —

Drawing Lewis Structures Worksheet

Lewis Dot Structure Practice Worksheet

Practice Drawing Lewis Structures Worksheets

Lewis Dot Diagram Practice

Drawing Lewis Structures Practice Worksheet

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Structure Practice Worksheet Lewis Dot Diagrams Chemistry Handout
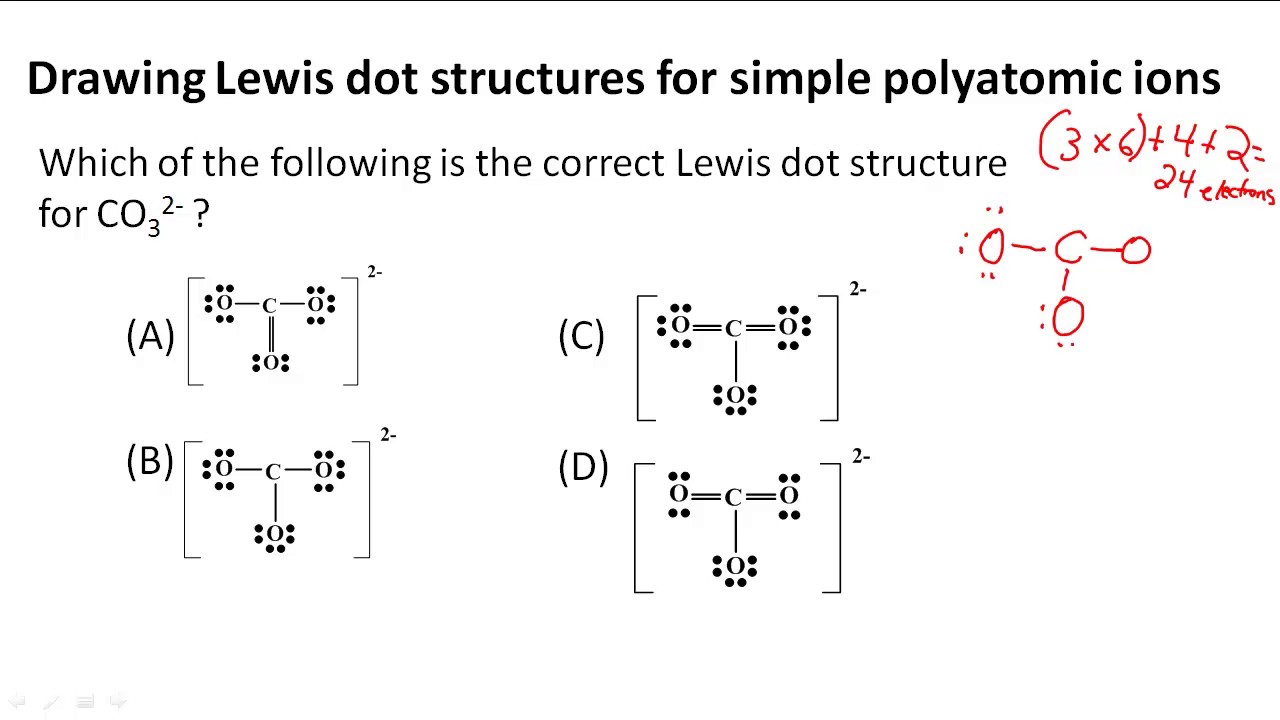
Lewis Structure Practice Worksheet
Web Lewis Structures Practice Worksheet Draw The Lewis Structures For Each Of The Following Molecules.
Web To Practice Drawing Lewis Structures For Various Covalently Bonded Molecules And Polyatomic Ions.
C 2 H 4 (One Double Bond), C 4 H 6 (Two Double Bonds), And C 4 H 6 (One Triple Bond).
Give The Name Of The Electronic Arrangement And The Name For The Molecular Geometry For Each Of The Species In Question #1.
Related Post: